Field Safety Notice (FSN): A2L SET WITH SIAMESE TUBING B50

Product: A2L SET WITH SIAMESE TUBING B50
FSCA – Identifier: 2302/47920/11
Type of Action: RECALL DEVICE
Description of the Problem:
This recall has been initiated by the legal manufacturer, AMS – Anaesthetic Medical Systems:
Description of the product problem: A manufacturing deviation has given rise to an increased risk of leaking from the hub (see diagram below for likely leak location)
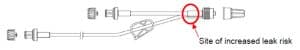
Hazard giving rise to the FSCA: If a risk is undetected by the user during priming or before use, there is an increased risk of under-infusion of the intended medicinal substance.
Affected Devices: Product Code – Batch/Lot – NHSSC Code
- VAN7220BL – 2287314A – FSB1336
Action Instructions:
Advice on action to be taken by Medical Staff
- Identify and quarantine any remaining stock of the affected product codes and batch/lot numbers.
- If you do not have stock from the affected batch number, stated above, mark the according checkbox on the enclosed FSCA Email Back Form, complete the form and send it to the following email address: technical@vygon.co.uk
- If you have stock from the affected batch number, stated above, mark the according checkbox on the enclosed FSCA Email Back Form, provide the additional information requested and send the completed form to the following email address: technical@vygon.co.uk
- On receipt of the completed FSCA Email Back Form, a Vygon (UK) Ltd. representative will contact you to arrange return of the affected stock and replacements.
- Please complete these actions by 1st March 2023.
Instruction for Distributors of affected batch numbers:
- Please identify and quarantine any remaining stock of the affected product codes and batch/lot numbers in your warehouse(s), complete the FSCA Email Back Form and send the completed form to technical@vygon.co.uk. On receipt of the completed FSCA Email Back Form, a Vygon (UK) Ltd. representative will contact you to arrange return of the affected stock and replacements.
- As a distributor, please provide this Field Safety Corrective Action (FSCA) to all your customers who have received stock of the affected product codes and batch numbers, as stated above. Please send your customers the following documents: A copy of this Field Safety Corrective Action, A copy of the FSCA Email Back Form
- The FSCA Email Back Form should be completed by your customer and returned to you.
- As a distributor you are required to confirm to Vygon (UK) Ltd that you have completed the instructed activity for all of your customers affected by this Field Safety Corrective Action. Please provide confirmation via email to technical@vygon.co.uk.
- Please complete these actions by 1st March 2023.
Vygon (UK) Ltd
Vygon (UK) Ltd has informed all customers, employees of Vygon (UK) Ltd and distributors of this Field Safety Corrective Action.
Transmission of this Field Safety Corrective Action
This notice needs to be passed on to all recipients/users of this product within your organisation, in particular, the following:
- All departments/wards
- Theatres
- MHRA Liaison Officer
- Risk Managers
- Medical Device Safety Officer
- Medical Directors
- Nursing Directors
- Procurement
Please consider end users, clinicians, risk managers, supply chain/distribution centers etc. in the circulation of this FSCA.
Please maintain awareness of this FSCA and resulting action for an appropriate period to ensure effectiveness of the corrective action.
Field Safety Notice (FSN):
Please find attached a copy of the FSN and email back form through this link.
The FSN has been sent to all customers of the affected codes.
For more information please use the Contact Form
Field Safety Notice: ACCUFUSER 300ML 10ML/HR TA
Contact Us
For more information on this field safety notice, or any other queries please get in touch: