Home / Obstetrics, Neonatology & Enteral / Neonatology: Safe Enteral feeding
Why do we need a safe enteral system?
Did you know before ISO 80369, 40% of misconnections were related to the Enteral route?
ISO 80369-1 was introduced in January 2011 and provides general requirements for these connectors, to prevent misconnections with IV lines and other medical equipment.
“...Misconnections of enteral devices may be underreported because connecting feeding tubes to the wrong tubing, such as an I.V. or dialysis catheter, is often attributed to human error rather than device failure. ….”(1)
Reduce—and report—enteral feeding tube misconnections
Enteral nutrition for preterm babies: risks to be aware of
Because of their physiological immaturity, preterm babies have specific needs, which means they require medical devices adapted to meet these needs.
The insertion and use of gastric tubes is one of the most common medical procedures in the neonatal intensive care unit. These tubes are used for gastric decompression, enteral nutrition and drug administration.
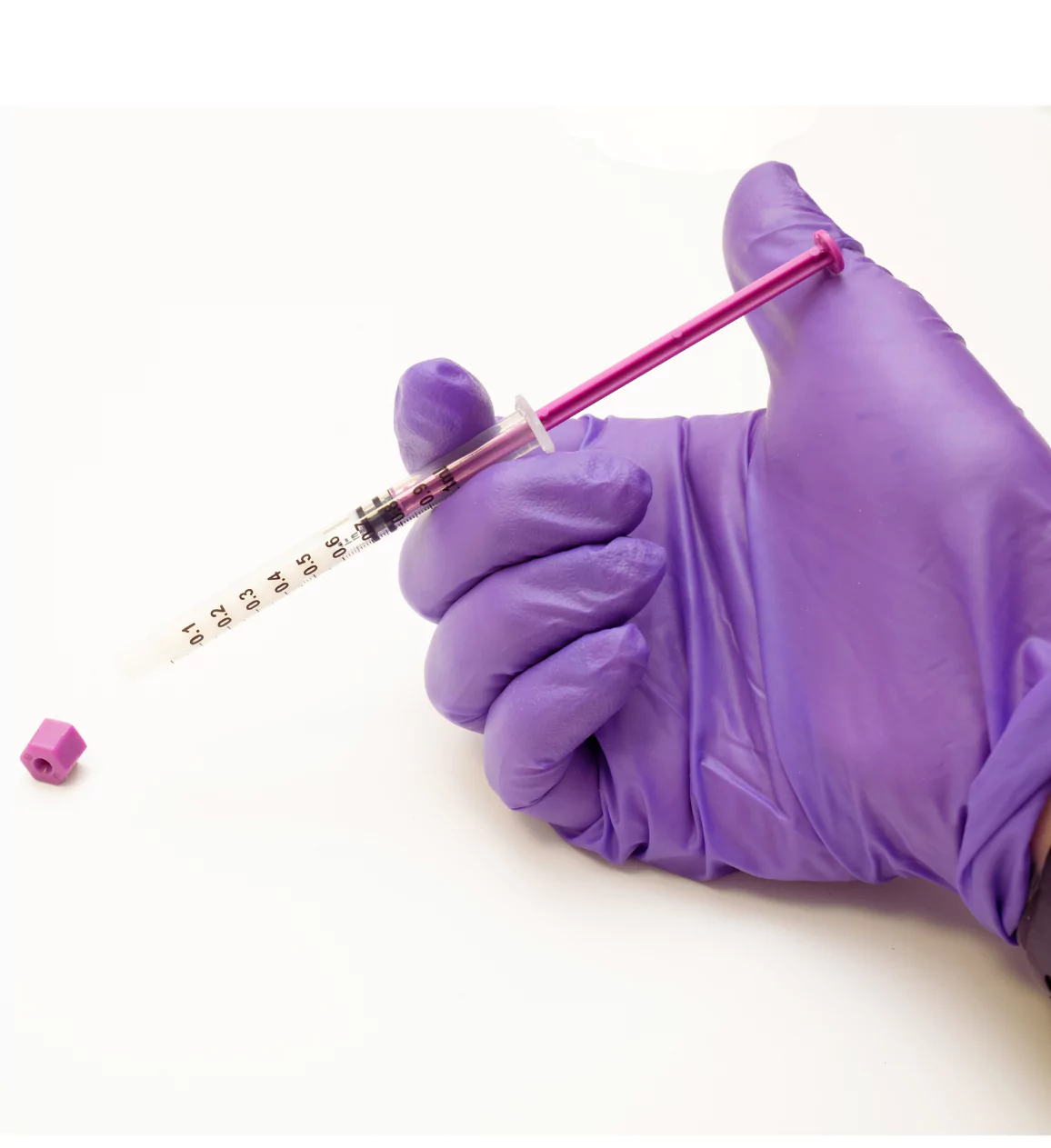
Calculations and dosage:
The therapeutic margin of a drug is the difference between the optimal effective dose and the dose at which unacceptable adverse effects occur.
When dealing with doses as low as 0.03ml for a 500g neonate, the therapeutic margin is minimal. This is because even the slightest variation in drug dosage is potentially dangerous for preterm patients, and can lead to complications such as respiratory distress, cardiovascular problems and increased drowsiness.
It is therefore necessary to use a system specially designed for neonatology.
Webinar
Comparison of Dosing Accuracy ENFit LDT vs neonatal specific ISO compliant syringe.
Webinar By Dr. O’Mara.
Avoiding contamination:
Neonates have a developing immune system which puts them at high risk of developing infections and not being able to fight them off.
Feeding tubes for newborns must never create a risk of bacterial contamination via the enteral route.
However, a study has shown that necrotising enterocolitis (NEC) develops in 5-10% of preterm babies in association with enteral feeding and bacterial colonisation.(1)
We recommend using tubes with small connectors meant specifically for neonatology.
(1) Biofilm-based Healthcare-associated Infections - 2011 - Cilieborg MS, Boye M, Sangild PT. Bacterial colonization and gut development in preterm neonates. Early Hum Dev. 2012 Mar;88 Suppl 1:S41-9. doi: 10.1016/j.earlhumdev.2011.12.027. Epub 2012 Jan 28. PMID: 22284985.
COMING SOON: we are excited to continue working with Dr O’Mara as she releases her latest study, investigating the Impact of Design on Neonatal Enteral Device Systems on Contamination.

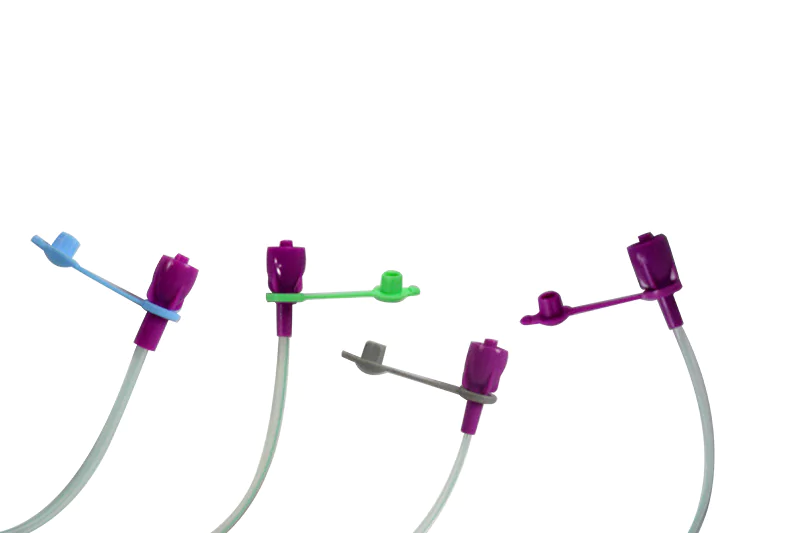
Benefits of Nutrisafe2™

Increased patient safety
Nutrisafe2 is not only designed specifically for neonatal patients, but the patented range complies with ISO 80369-1 reducing the risk of connection errors while being adapted to infant physiology.
The system can be screwed into place, preventing accidental disconnection and ensuring that the patient receives the nutrients and drugs being administered.

Mitigate risk of dosing variances
These dedicated connectors have 70% less deadspace when compared to the ENFit™/ISO 80369-3 standard, improving accuracy and minimising the risk of drug over- or under-dosing.

Cleaner devices
The other benefit of its small design is Nutrisafe2 is less prone to keep residues.
The smaller connector design has a less threaded interior, this combined with the reduced dead space reduces the risk of sequestering milk or medication and reducing the risk of infection.
Nutrisafe2™ product range
Specially designed for neonatal patients and newborns, the PVC nutrisafe2 feeding tubes are intended to be used for short-term (up to 5 days). For medium-term (1 to 4 weeks), PUR nutrisafe2 feeding tubes and silicon gastro-duodenal feeding tubes will fulfill your needs.
Safe enteral feeding syringes with a Nutrisafe2 connection designed for neonates and children.
There are numerous Nutrisafe2 accessories available such as: extension tubes, syringe accessories, sampling devices, caps, adaptors, securement devices, and stopcocks. Ensuring you have everything you need to hand and ready to use with Nutrisafe2 connectors.
For more information please contact us
Vygon UK is here to support you, your clinicians, and your Trust in your transition to Nutrisafe2™. Vygon UK is on hand with support for every step of the journey so get in touch today to find out more.