Urgent Field Safety Notice – Easymoov6 Enteral Feeding Pump

Urgent Field Safety Notice – Easymoov6 Enteral Feeding Pump
For Attention of*: Identify either by name or role who needs to be aware of the hazard and/or take action. If this is multiple recipients then include full list.
Contact details of local representative (name, e-mail, telephone, address etc.)*
Technical Department – technical-uk@vygon.com, Tel: 01794 748800 – Vygon (UK) Ltd, The Pierre Simonet Building, V Park, Gateway North, Latham Road, Swindon, SN25 4DL
FSN Ref: 2507/51788/00 – PM6/SS14/FSN FSCA Ref: 2507/51788/00 – PM6/SS14/FSN
Urgent Field Safety Notice (FSN)
Device Commercial Name
Risk addressed by FSN
1. Information on Affected Devices*
1. Device Type(s)* – Enteral feeding pump
2. Commercial name(s) – Easymoov6
3. Unique Device Identifier(s) (UDI-DI)* – Reference – 0VEPM6G02 UDI-ID – 03660812096553
4. Primary clinical purpose of device(s)* – Enteral feeding
5. Device Model/Catalogue/part number(s)* – See reference 1.3
6. Software version – All Software versions
7. Affected serial or lot number range – All serial numbers
8. Associated devices – N/A
2. Reason for Field Safety Corrective Action (FSCA)*
1. Description of the product problem* – The legal manufacturer, Medwin, has been informed of an incident in a healthcare facility. The user failed to place the silicone tubing around the rotor, resulting in significant overflow.
2. Hazard giving rise to the FSCA* – Risk of overflow and free flow
3. Probability of problem arising – This is the second report of this nature, which can also arise from incomplete user training.
4. Predicted risk to patient/users – Overfeeding, Digestive disorders
5. Further information to help characterise the problem – N/A
6. Background on Issue – The legal manufacturer received two reports of this nature.
7. Other information relevant to FSCA – N/A
3. Type of Action to mitigate the risk*
1. Action To Be Taken by the User*
☐ Identify Device
☐ Quarantine Device
☐ Return Device
☐ Destroy Device
☐ On-site device modification/inspection
☐ Follow patient management recommendations
☐ Take note of amendment/reinforcement of Instructions For Use (IFU)
☒ Other
☐ None
It is advisable to remind users, of the correct way to install the tubing on the enteral nutrition pump.
Please refer to the instructions for use in appendix 1.
A new cover with an added security has been designed and Vygon (UK) Ltd will arrange to replace the cover of Easymoov6 pumps in the UK.
2. By when should the action be completed? – As soon as possible
3. Particular considerations for: N/A
Is follow-up of patients or review of patients’ previous results recommended? N/A
4. Is customer Reply Required? * (If yes, form attached specifying deadline for return) – Yes
5. Action Being Taken by the Manufacturer
☐ Product Removal
☐ On-site device modification/inspection
☐ Software upgrade
☐ IFU or labelling change
☒ Other
☐ None
Vygon (UK) Ltd will arrange to replace the cover of Easymoov6 pumps in the UK.
6. By when should the action be completed? – As soon as possible
7. Is the FSN required to be communicated to the patient /lay user? – Yes
8. If yes, has manufacturer provided additional information suitable for the patient/lay user in a patient/lay or non-professional user information letter/sheet? – See appendices
3. General Information*
1. FSN Type* – Initial
2. For updated FSN, reference number and date of previous FSN – N/A
3. For Updated FSN, key new information as follows: Remind of the instructions to set up the enteral feeding set on the rotor. And information about the new cover with an added security.
4. Further advice or information already expected in follow-up FSN? * – N/A
5. If follow-up FSN expected, what is the further advice expected to relate to: – N/A
6. Anticipated timescale for follow-up FSN – N/A
7. Manufacturer information (For contact details of local representative refer to page 1 of this FSN)
a. Company Name – Vygon (UK) Ltd (on behalf of MEDWIN France)
b. Address – The Pierre Simonet Building, V Park, Gateway North, Latham Road, Swindon, Wiltshire, SN25 4DL
c. Website address – www.vygon.co.uk
8. The Regulatory Authority of your country has been informed about this communication to customers. *
9. List of attachments/appendices: – Appendix 1: Instructions for use (set-up of the enteral feeding set) Appendix 2: Cover change Acknowledgement of receipt
10. Name/Signature – Kate O’Connell, Technical Support Manager (On behalf of Jérémy Imbert, Quality Manager)
Transmission of this Field Safety Notice
This notice needs to be passed on all those who need to be aware within your organisation or to any organisation where the potentially affected devices have been transferred. (As appropriate)
Please transfer this notice to other organisations on which this action has an impact. (As appropriate)
Please maintain awareness on this notice and resulting action for an appropriate period to ensure effectiveness of the corrective action.
Please report all device-related incidents to the manufacturer, distributor or local representative, and the national Competent Authority if appropriate, as this provides important feedback..*
Appendix 1: Reminder on tubing installation on Easymoov6 enteral feeding pumps
To install the tubing, follow the steps below:
• Connecting the tubing to the nutrient bag:
• After checking the integrity of the packaging, remove the tubing from the bag.
• Connect the tubing to the nutrient bag and suspend the bag.
• Install the tubing on the pump and bleed it in accordance with the table below taken
from the operating instructions:
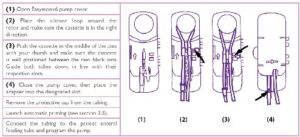
Please note:
• not to connect the tubing to the probe before priming,
• to check the correct position of the silicone tube around the rotor:

Appendix 2: Security added to the cover















