Qimono the complete closed system for safe delivery of cytotoxic drugs
Stay safe with Qimono
It is widely documented that exposure to Systemic Anti-Cancer Therapy (SACT), can pose a significant health risk to patients during treatment as well as healthcare workers involved in the preparation and administration of the drugs .
Many SACT agents are known to be carcinogenic, teratogenic and mutagenic. Exposure may be through skin contact, skin absorption, inhalation of aerosols and drug particles as well as ingestion and needlestick injuries. This can occur at various points of the treatment process: during the preparation of the medication, whilst the drugs are administered and after therapy .
Adverse health effects reported by chemotherapy nurses include headaches, dizziness, nausea and hair loss.
Description of the device
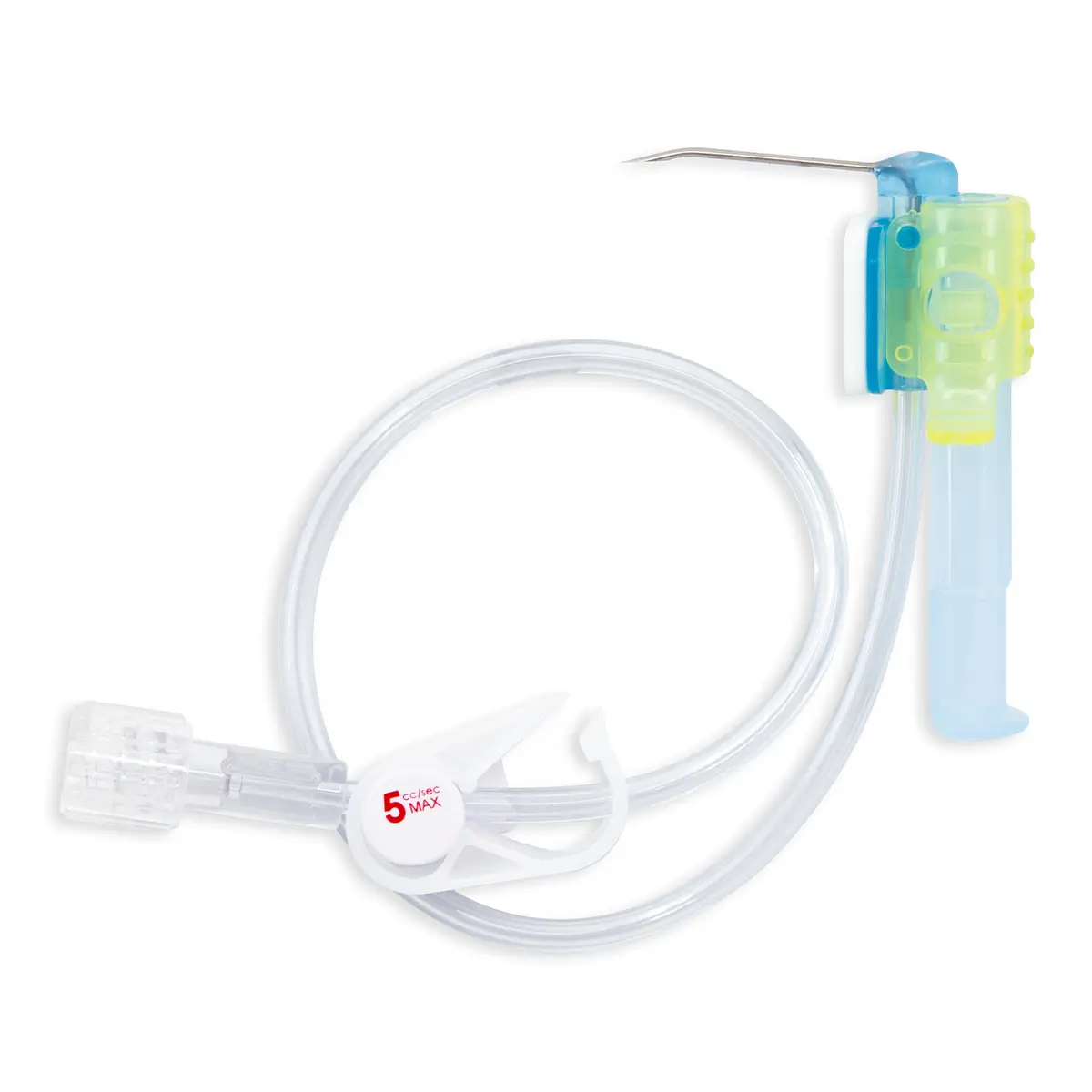
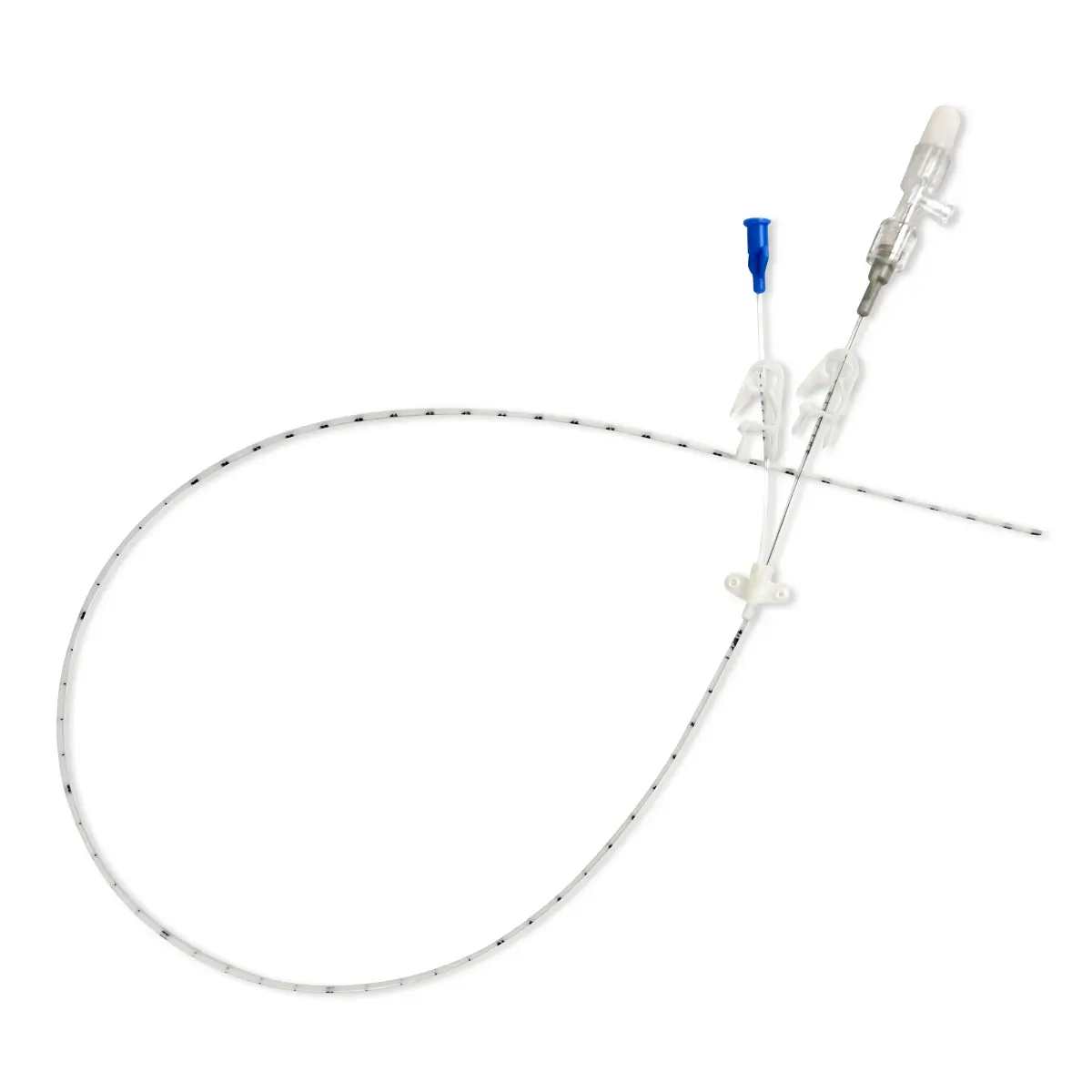
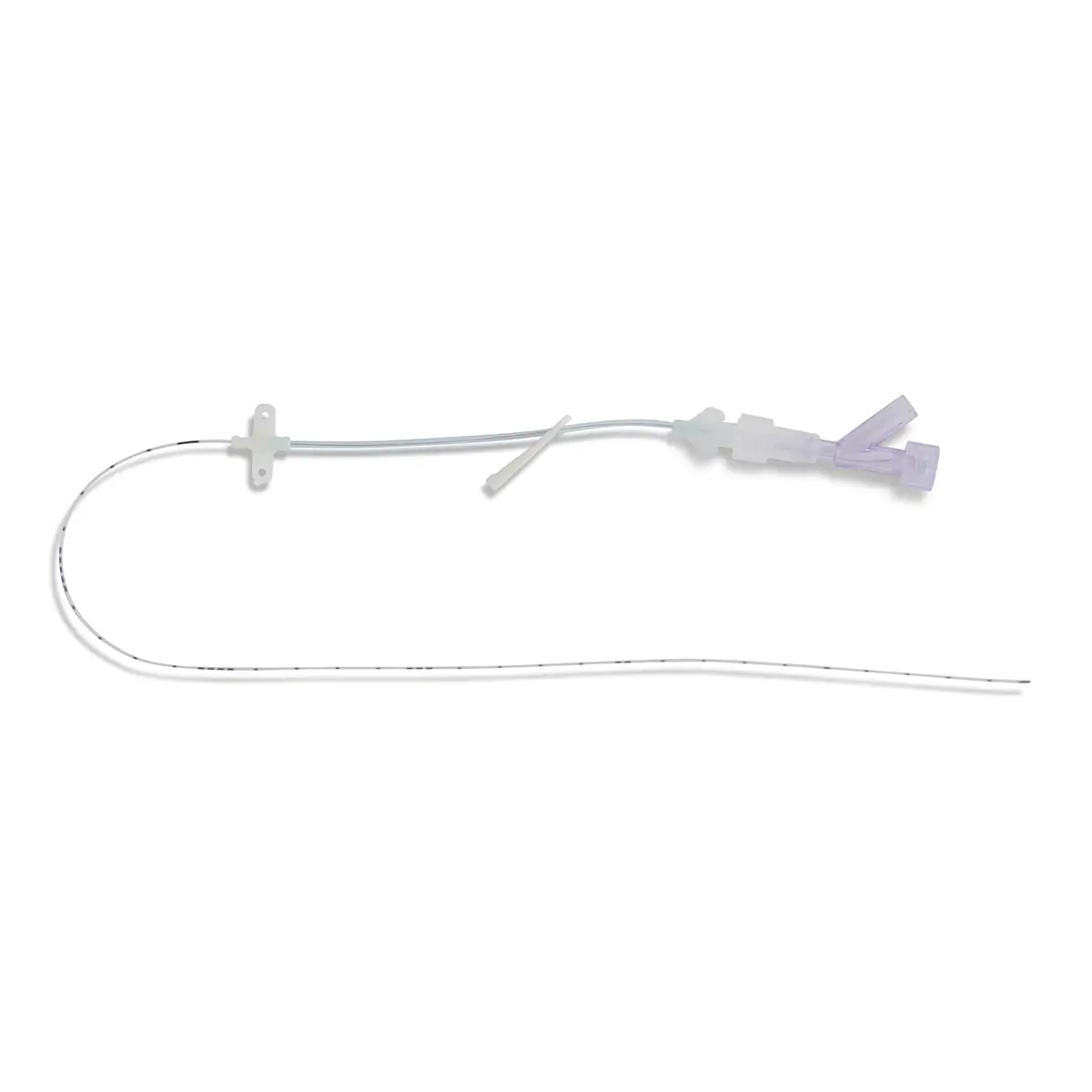
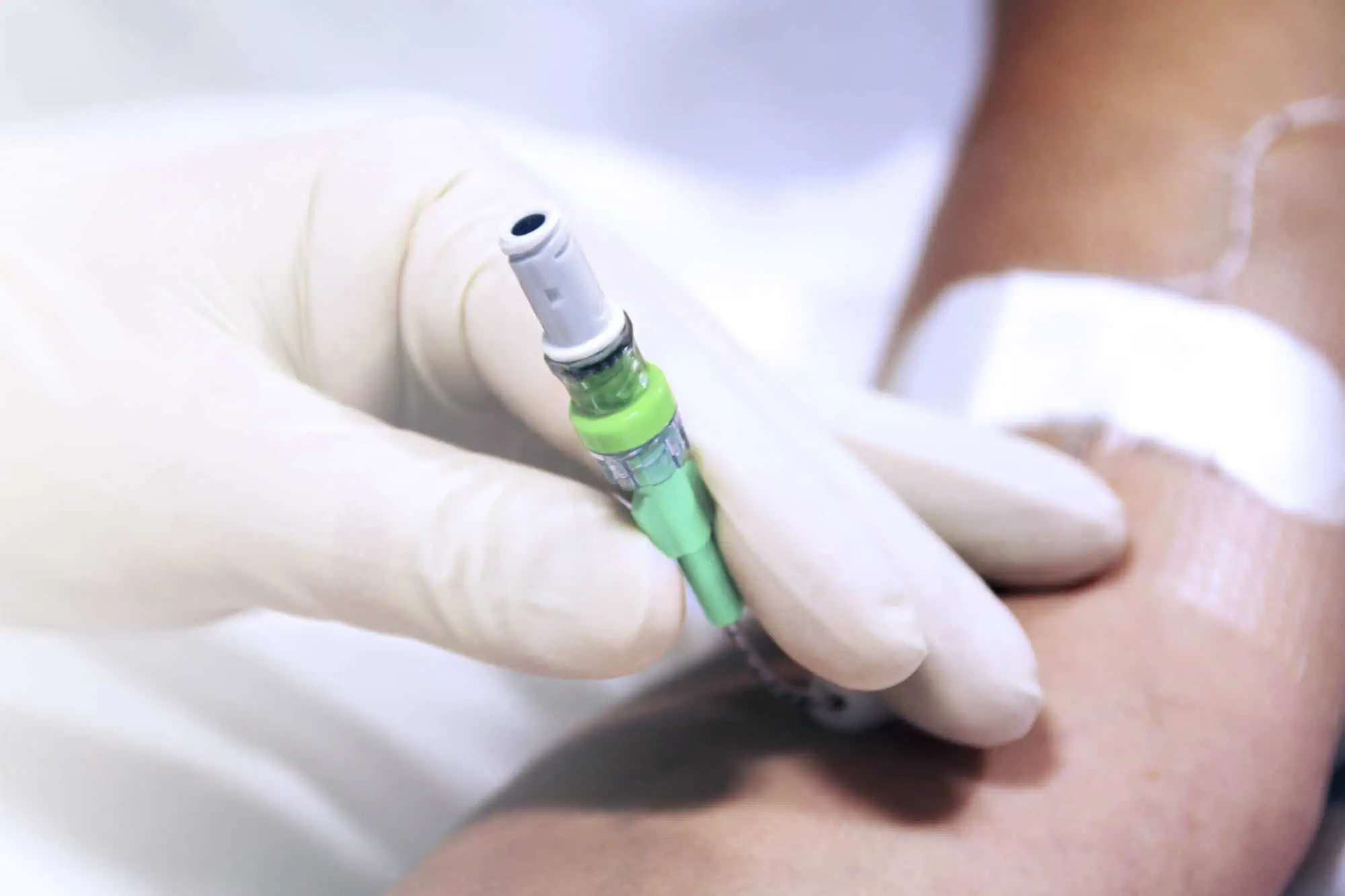
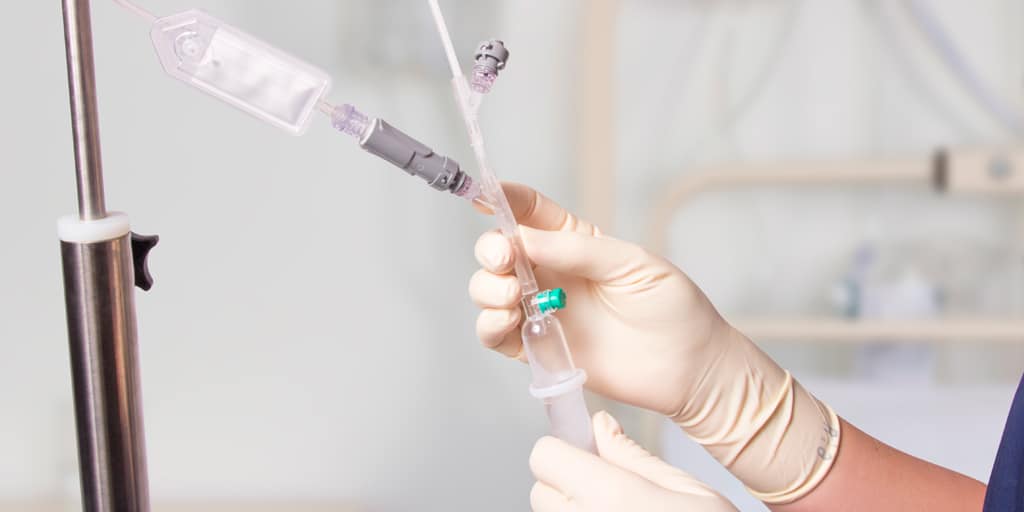
Qimo Female is a multi-purpose, closed, needle-free, I.V. connection device for blood sampling, intermittent injections or continuous infusions of fluids or drugs. Qimo Female has a very easily disinfectable membrane (disinfection must be carried out before and after use) which closes automatically when the infusion line or the syringe is disconnected.
At the distal end, Qimo Female has a male Luer-lock connection allowing it to be connected to a venous catheter or an I.V. accessory with a female Luer-lock hub (intravenous catheter, extension tube, Huber needle for infusion in an implantable port, stopcock, ramp of stopcocks, spike …).
At the proximal end, Qimo Female has a female Luer-lock end, closed thanks to a membrane in polyisoprene.
When a venous catheter or an I.V. accessory is fitted with a Qimo Female, Qimo Female can be connected onto:
– the Luer bayonet of Qimo Male (mounted on a syringe, an extension tube or an infusion set),
– a male Luer-lock end (or Luer Slip) of a syringe or an extension tube.
When a Qimo Male or a male Luer end is connected onto Qimo Female, the membrane in polyisoprene moves thanks to a metallic spring and opens an internal conduit allowing the injection, infusion, sampling.
When the device is disconnected, the spring pushes back the membrane and automatically closes the device.
Note: for the users who know Vygon Bionector, please note that Qimo Female is in fact a Bionector. The difference lies in the ring with notches allowing to receive the lugs of Qimo Male. Otherwise Qimo Female works like a Bionector.
Qimo Female is resistant to lipid emulsions and antiseptics.
Qimo Female is approved for use with power injectors (CT-scan).
Maximum pressure resistance : 350 psi (24 bars).
To inject contrast medium products (Visiopaque 320 mg iodine/ml), the flow rate has been measured as such:
35 psi (2.4 bars) : > 5 ml/s
145 psi (10 bars) : > 10 ml/s
Replacement frequency: < 360 connections or 7 days
Priming volume: 0.03 ml
Flow rate: 105 ml/min