Qimono the complete closed system for safe delivery of cytotoxic drugs
Stay safe with Qimono
It is widely documented that exposure to Systemic Anti-Cancer Therapy (SACT), can pose a significant health risk to patients during treatment as well as healthcare workers involved in the preparation and administration of the drugs .
Many SACT agents are known to be carcinogenic, teratogenic and mutagenic. Exposure may be through skin contact, skin absorption, inhalation of aerosols and drug particles as well as ingestion and needlestick injuries. This can occur at various points of the treatment process: during the preparation of the medication, whilst the drugs are administered and after therapy .
Adverse health effects reported by chemotherapy nurses include headaches, dizziness, nausea and hair loss.
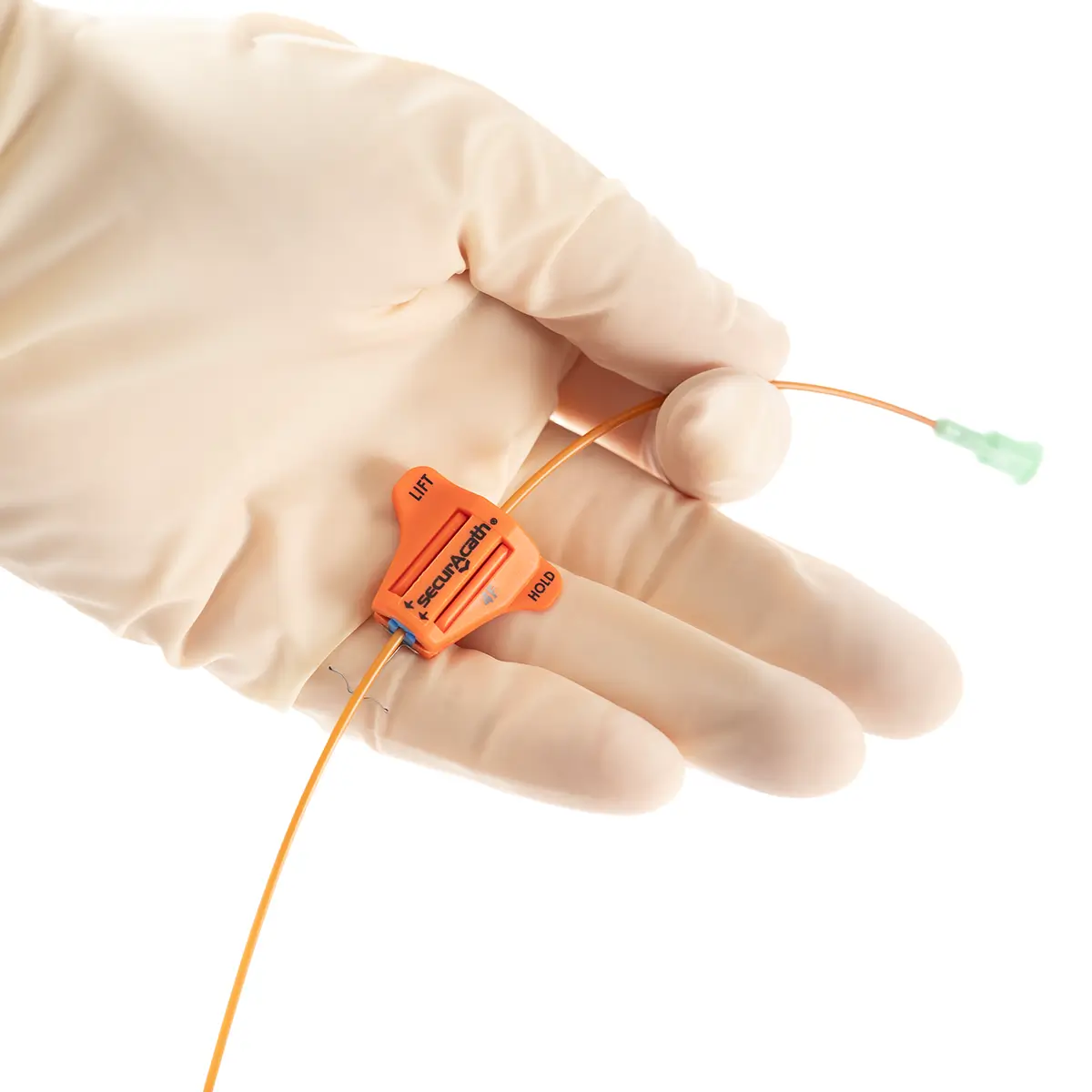
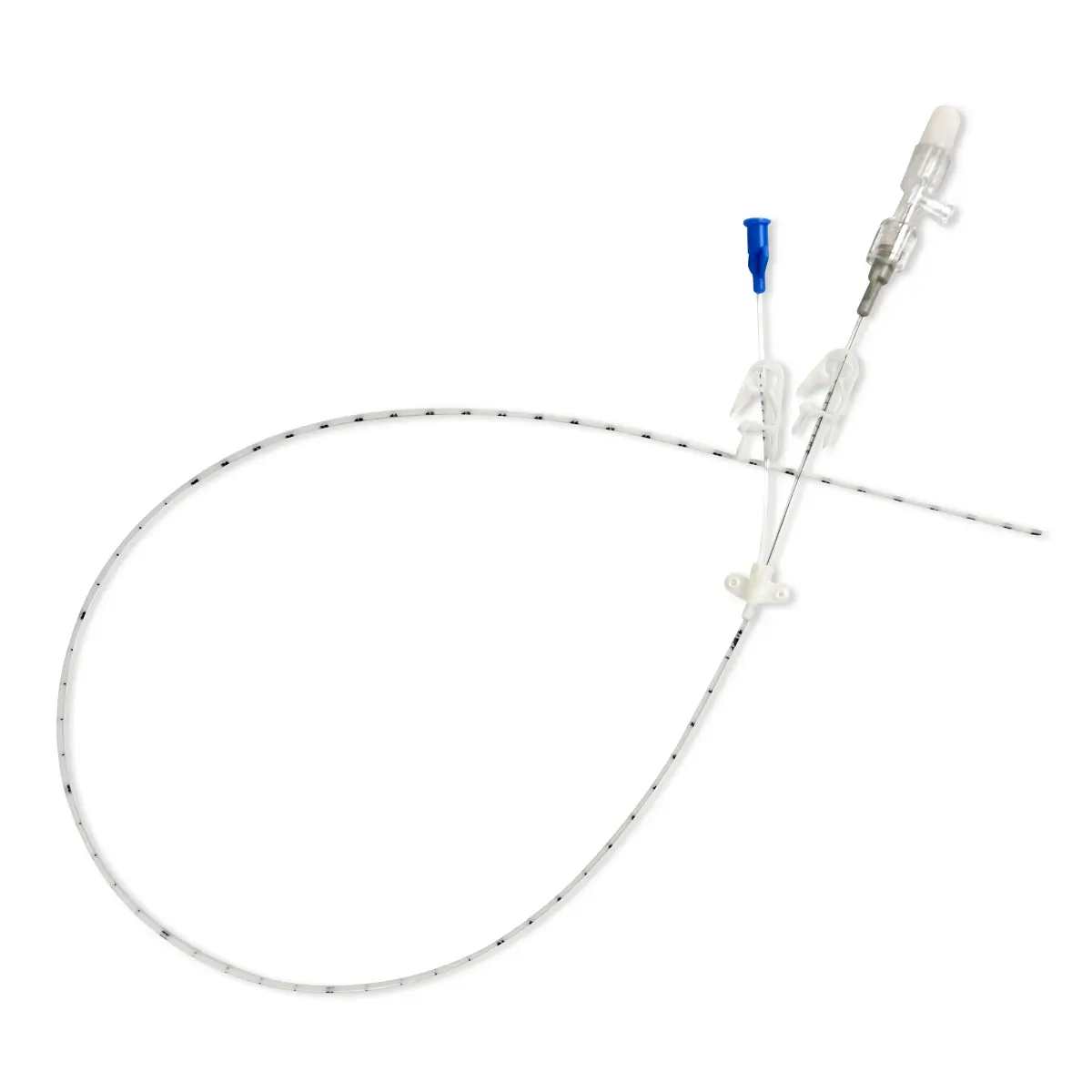
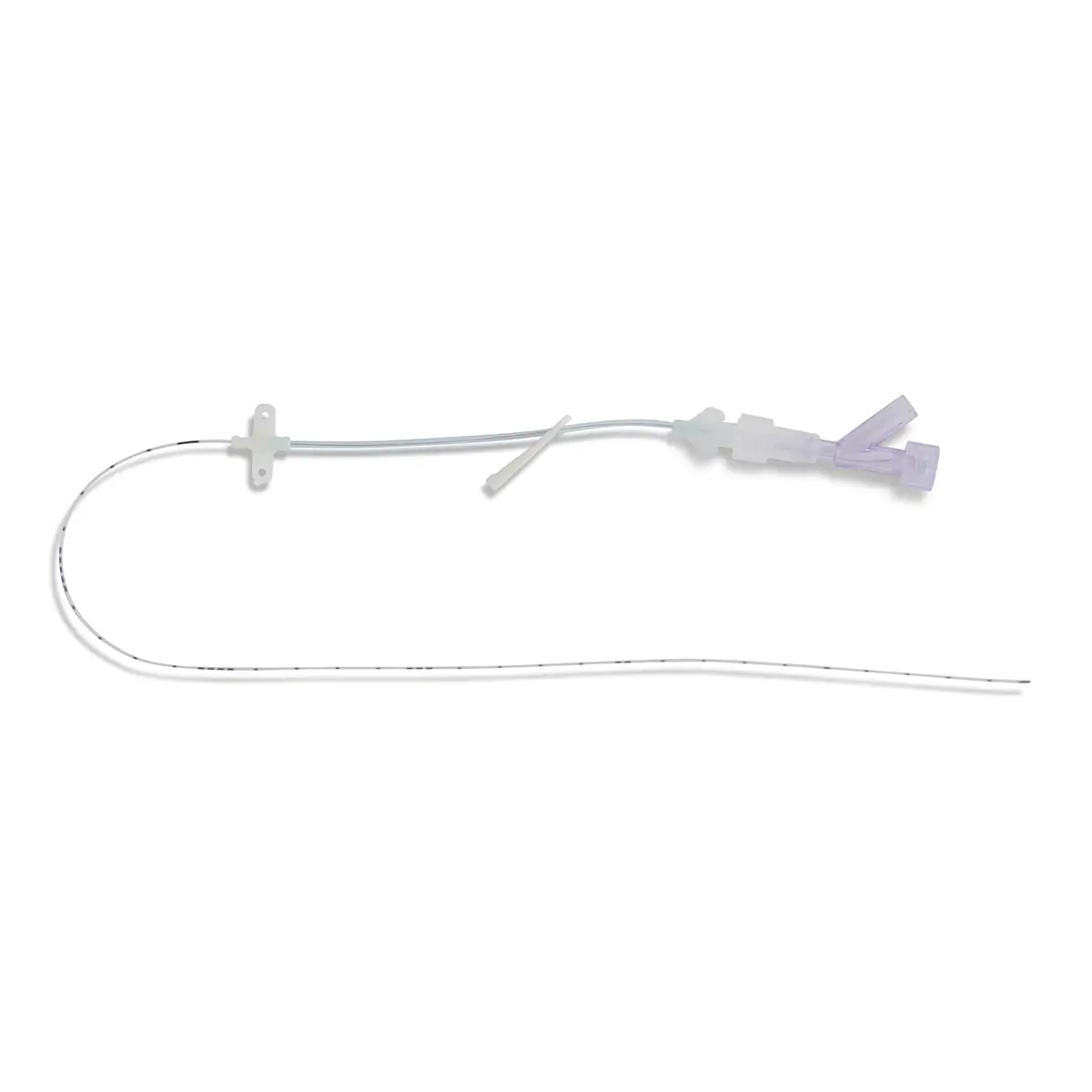
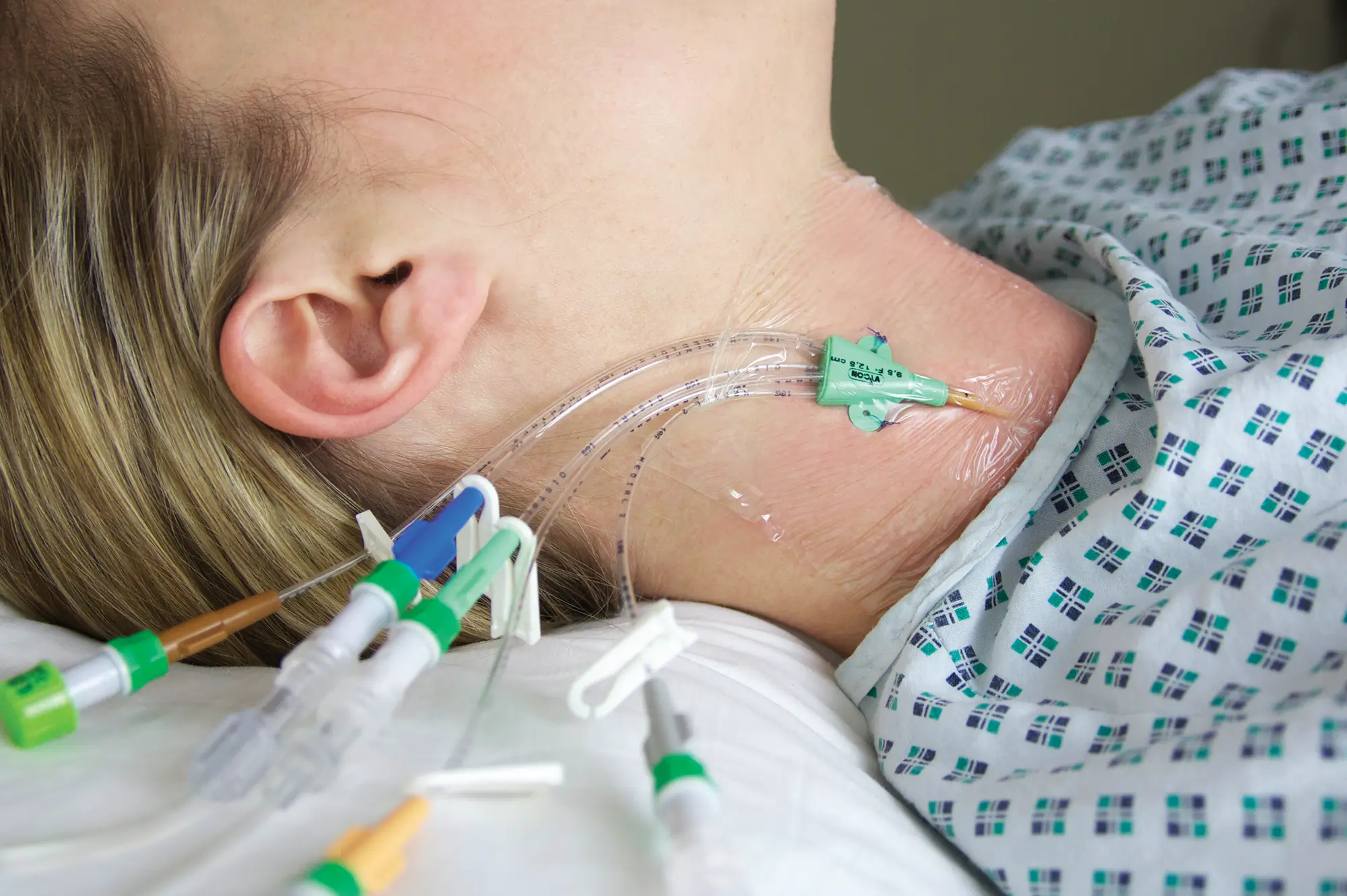
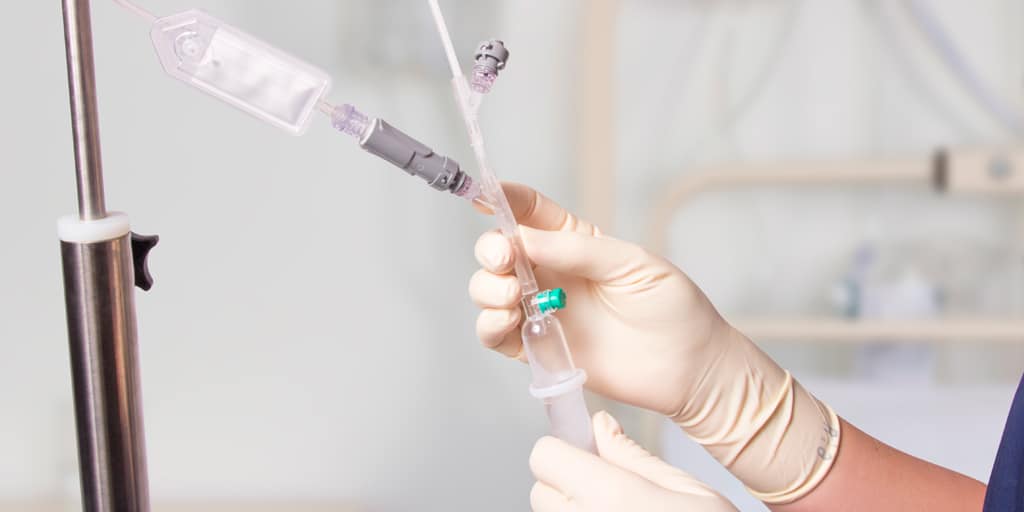
QimoOctopus with Qimo Female and Qimo Male is a multi-purpose catheter accessory, a double lumen IV extension tube with needleless connectors allowing intermittent injections or continuous infusions of fluids or medications.
IV extension tube in flexible, transparent polyurethane tubing with a low priming volume.
One lumen of QimoOctopus is fitted with a non-return valve, the other lumen is fitted with a Qimo Female connector.
The Qimo Male must be connected at the distal end of QimoOctopus.
QimoOctopus has the following advantages:
– Eliminates the risk of access-associated needle-stick injury.
– Reduces the risk of cannula movement in the vein causing mechanical vein irritation.
– Negates the need to use the cannula injection port (non disinfectable), Qimo Female is disinfectable.
– Eliminates the need to use a 3-way tap to run simultaneous compatible solutions.
– Provides closed system (avoiding blood reflux) when accessing the catheter.
– PUR material (no PVC, phthalate-free, no release of plasticizers).
Qimo Female is a multi-purpose, closed, needle-free, connection device with disinfectable membrane. See the corresponding technical file code 7210.02.
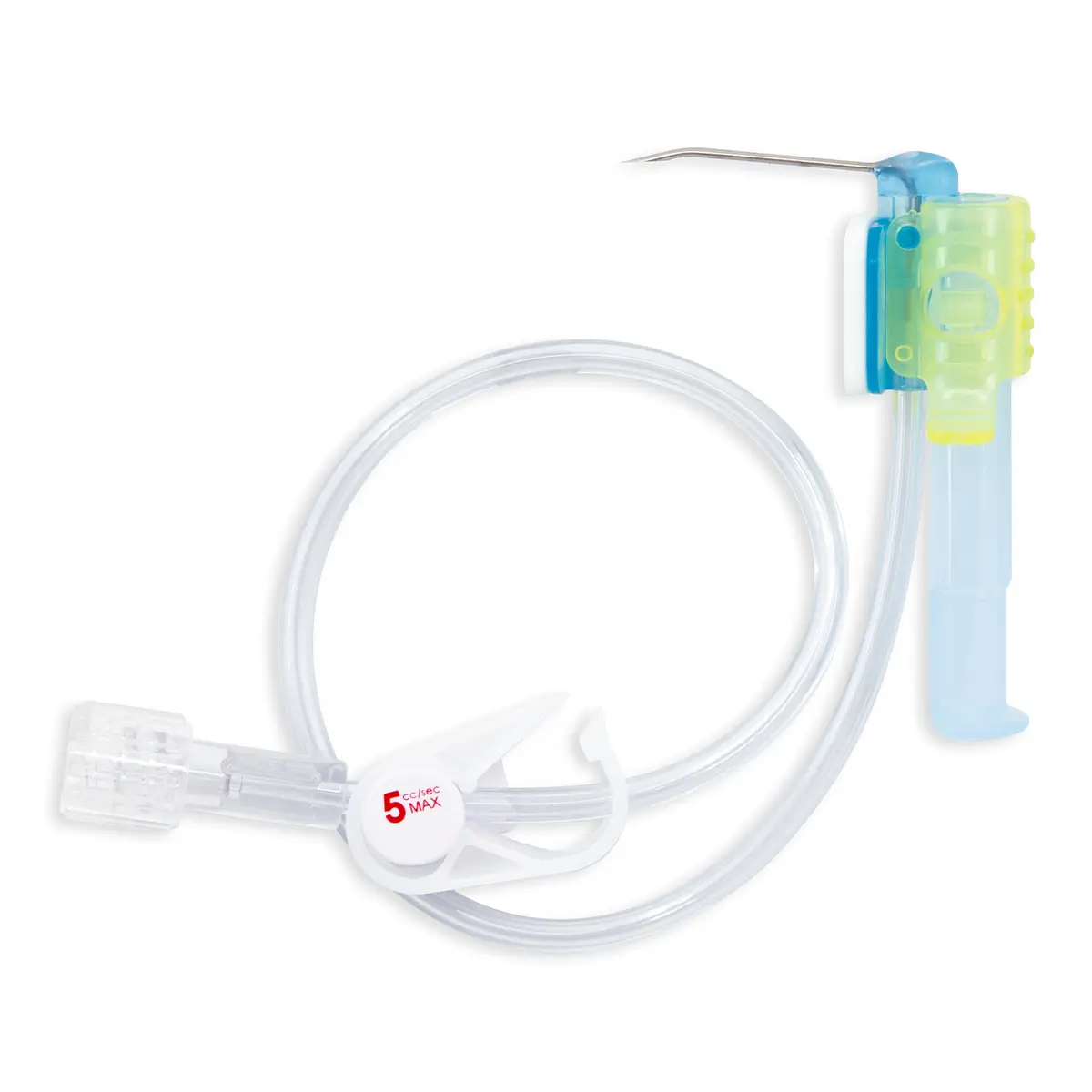
Qimo Male is a multi-purpose, closed, needle-free, I.V. connection device which has been designed for the preparation and safe administration of hazardous drugs. See the corresponding technical file code 7210.91.
QimoPrime is priming device. It is used to prime Qimo Male after Qimo Male has been attached onto the distal end of QimoOctopus. See the corresponding technical file code 7210.81.