Qimono the complete closed system for safe delivery of cytotoxic drugs
Stay safe with Qimono
It is widely documented that exposure to Systemic Anti-Cancer Therapy (SACT), can pose a significant health risk to patients during treatment as well as healthcare workers involved in the preparation and administration of the drugs .
Many SACT agents are known to be carcinogenic, teratogenic and mutagenic. Exposure may be through skin contact, skin absorption, inhalation of aerosols and drug particles as well as ingestion and needlestick injuries. This can occur at various points of the treatment process: during the preparation of the medication, whilst the drugs are administered and after therapy .
Adverse health effects reported by chemotherapy nurses include headaches, dizziness, nausea and hair loss.
Description of the device
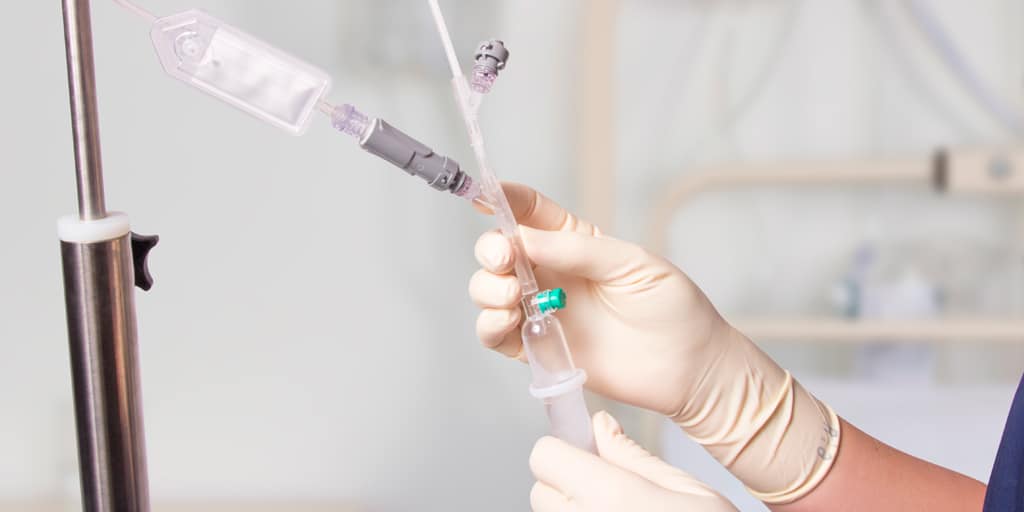
Needleless vial access cap for small vial cap 14 mm (codes 7219.01/010) or large vial cap 20 mm (codes 7219.02/020) with plastic spike.
After perforation of the cap of the vial, Qimo Female will avoid the contamination of the drug and will allow multiple vial accesses for drug sampling with a syringe after disinfection of the membrane of Qimo Female.
Qimo Female is a multi-purpose, closed, needle-free, I.V. connection device. See the corresponding technical file code 7210.02.
Codes 7219.01/02: QimoCap – 1 unit in a supple blister
Codes 7219.010/020: Set of 25 QimoCap
– Code 7219.010: 25 units in a supple blister / 100 units in a box / 1 800 units in a case
– Code 7219.020: 25 units in a supple blister / 100 units in a box / 1 200 units in a case