Improving Peripheral IV Cannulation Practices

An answer to the unintended consequences of peripheral IV cannulation.
The Why
Peripheral IV cannulation (PIVC) is the most prevalent procedure in healthcare (1) with circa 30 million cannulas used in the UK every year (ref NHSSC). The prevalence is significant as up to 80% of people that are admitted to a hospital will receive a cannula (3,4). The failure rate for PIVC is between 33%-69% (5,6). The reasons for failure are varied and include occlusion, infiltration, extravasation, phlebitis, dislodgement, pain, infection, and accidental removal (4).
Where are we now?
The Median dwell time for a PIVC is shown to be 28.5 hours (7). The result is the cannula will need to be re-sited so that treatment can continue. The burden of failed IV cannulation is high and multi-factorial and can lead to significant on-costs. These burdens include missed treatments, secondary treatment plans due to adverse effects, increased length of stay, and poor patient experience. Typically, a course of antibiotics can be prescribed for anywhere up to 14 days. Routine replacement of PIVCs increases healthcare costs, staff workload and requires patients to undergo repeated invasive procedures (8).
What’s next?
Where we find ourselves currently is a place of transition and improvement. There have been several studies and audits of practice carried out in recent years (4,5,7,8,10,12,13), however Helm et al (2015) stated that the reasons for failure could be attributed to practice and product (9). In his follow up in 2019, he stated that not much has changed but the issues are less accepted, and change is occurring.
Get It Right First Time
A focus can be placed on first time PIVC insertion success rate as it has been reported that a PIVC placed first time is less likely to fail due to complications (10). To place a device first time is vital to the longevity of the device as well as the success of the treatment, patient comfort and protecting the vasculature for potential future vascular access.
Mangar et al (11) reported a success rate of 82% when using the Seldinger technique versus 65% when using a direct technique such as cannulation. Van Loon et al (12) looked at the cost of PIV cannulation and demonstrated through an observational study that first time success cost €9.32 but dramatically increased when five attempts were needed, €65.34. This becomes highly pertinent when considering patients with difficult IV access (DIVA) with this same study asserting 2 in 10 patients will be classified as DIVA and proposed an improved A-DIVA scale (Adult Difficult Intra Venous Access). In a 2019 meta-analysis, Van Loon et al (2020) stated the literature reported up to a 30% first time failure rate for PIV cannulation (13).
The How – A Cog in the Wheel
Although the answer to failed PIV cannulation is based on many variables, not least device selection, training, education, awareness and listening to the patient voice, the use of existing tools to make decisions regarding approach to placement and the type of device needed may well save time, money and benefit the patient with reference to treatment, pain and anxiety.
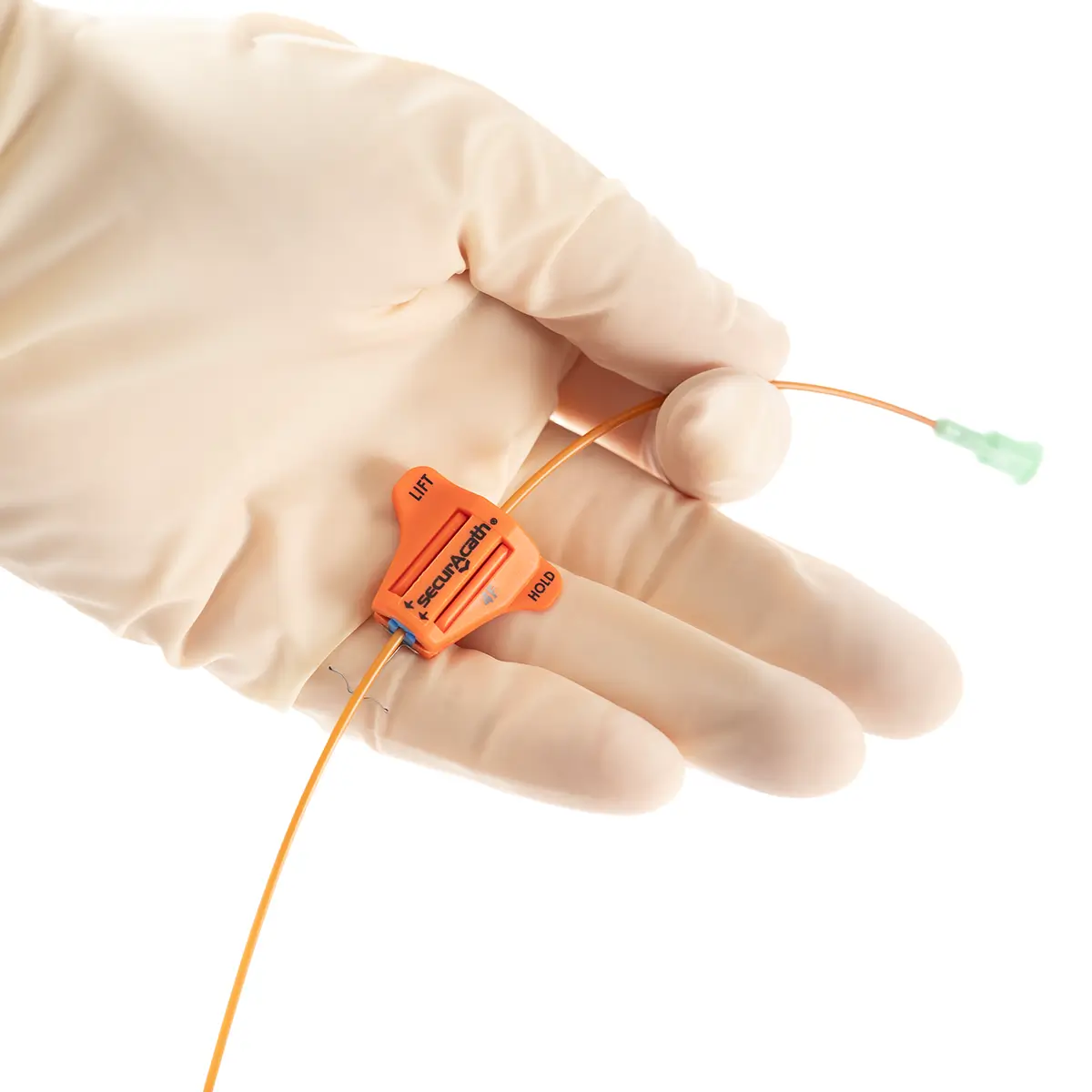
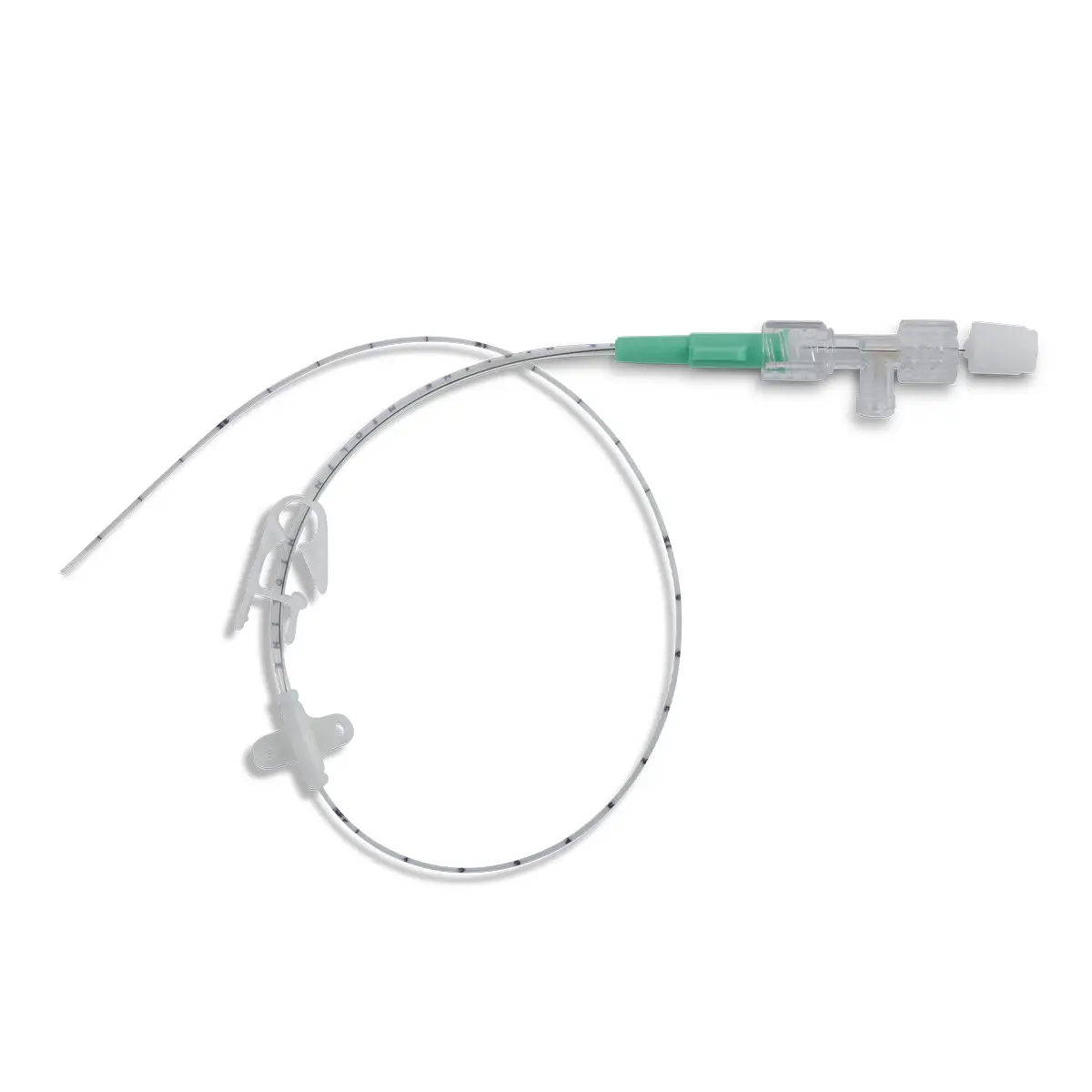
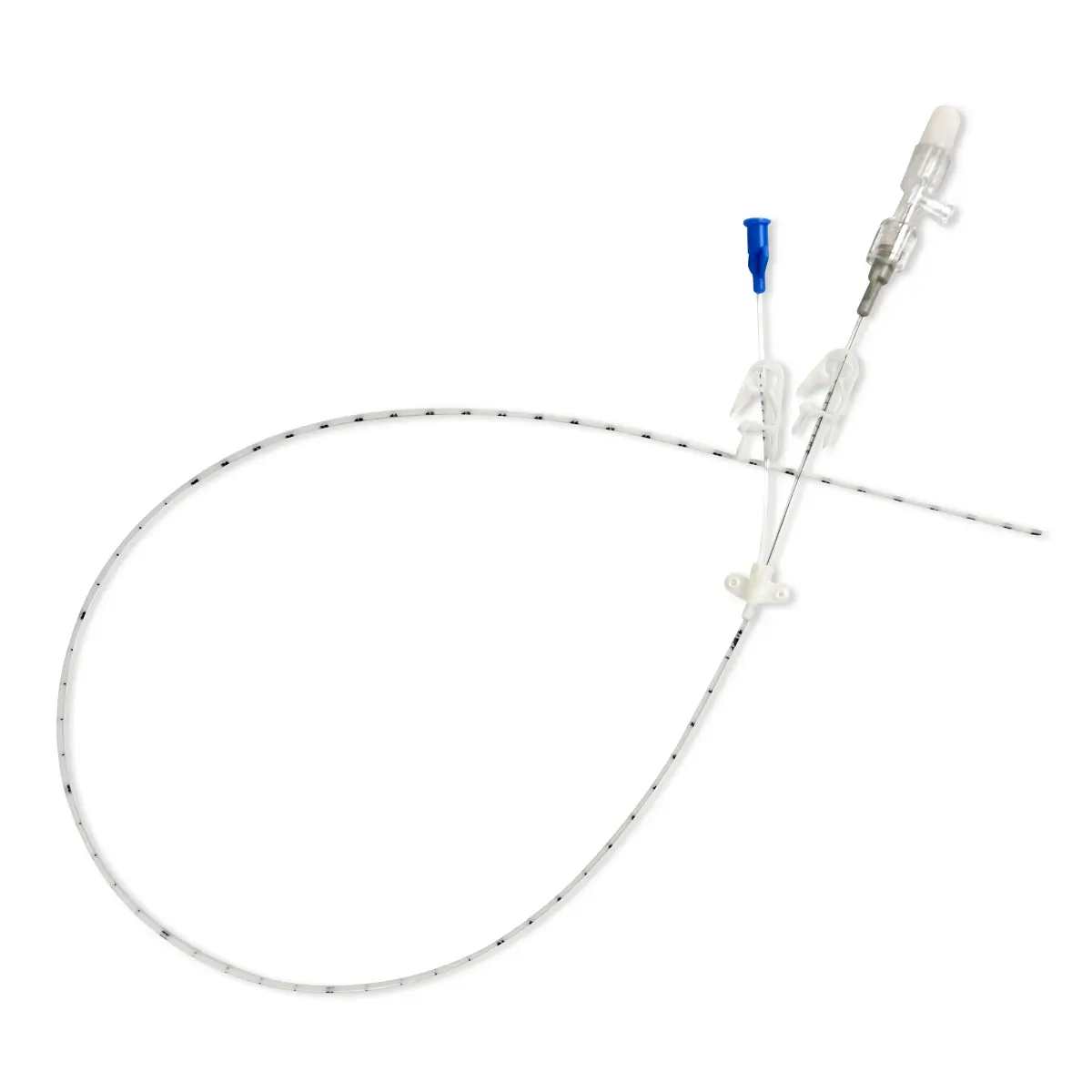
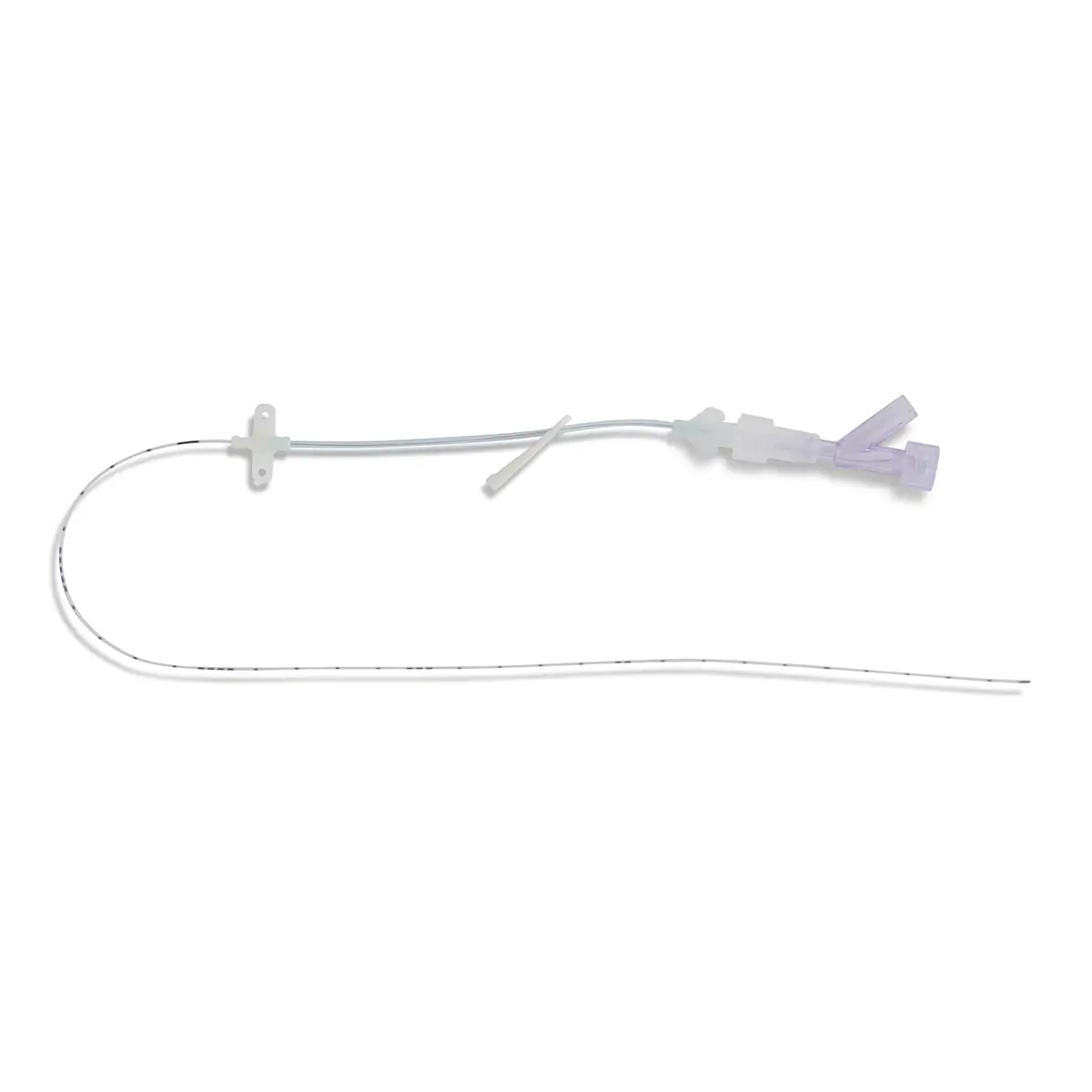
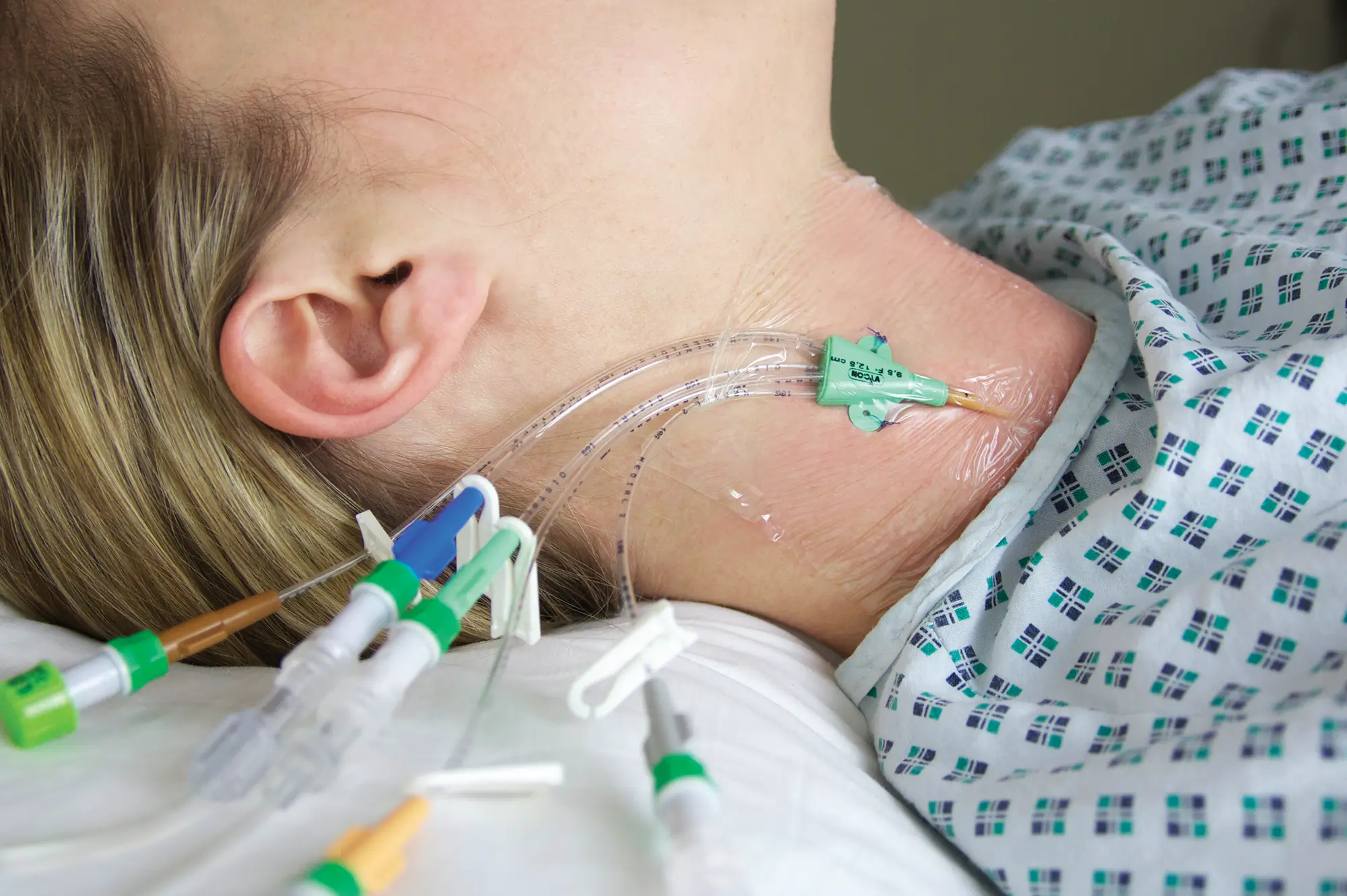
The use of Vygon’s extended dwell catheters/cannulas have been shown to provide a sustainable mode for peripheral vascular access, achieving a longer dwell time of between one and four weeks, and a higher first-time insertion rate due to the use of the Seldinger technique.
The nature of the Smartmidline’s thermosensitive material allows the catheter to sit comfortably in the vein, reducing the chance of vessel damage. Our power injectable Smartmidline has been designed in eight lengths and 4 gauges to provide the largest choice of catheters as we understand that one size does not fit all. Overall, this approach provides more sustainable and safer patient care.
Education & Training
The awareness and education surrounding IV access devices is critical to changing the attitudes and outcomes in peripheral IV administration. When considering IV device selection and the background behind this, Vygon UK have developed the tools necessary to support decision making.
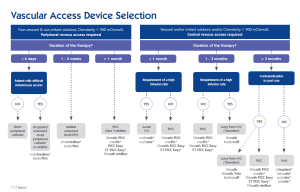
The vascular access device selection matrix separates peripheral vs central access and the anticipated time period for access. This allows early decision making in line with the proposed patient pathway.
The vascular access triage tool has been designed as an aide to understand if a PIVC or extended dwell catheter is appropriate for the patient. This tool incorporates the Vessel Health and Preservation vein assessment tool to assess VA appropriateness.
These tools work hand in hand and are used to implement the change in thinking and practise needed to deliver the right device for the right patient at the right time.
To answer the true question of training, Vygon UK have built a full eLearning package for device placement and care and maintenance. The EDC, Midline and PICC packages are accredited by the Royal College of Nursing (RCN) and offer 5 CPD points. The care and maintenance package is accredited by CPD UK. This standardises the approach to training and level of competency required to make the introduction of this initiative clinically and patient safe, as the competency framework is fully evidence based.
At Vygon, we propose to look at the holistic view of the patient journey and the cause and effect of cannula failure. By using the tools described, it is possible to assess a patient who needs vascular access and select the most appropriate device for the patient journey. This will improve operational efficiency, save money via wasted product and nurse/clinician time, reduce the amount of waste produced per patient journey and increase the satisfaction of patients and staff.
References
- Zingg, W. and Pittet, D. (2009) Peripheral Venous Catheters: An Under-Evaluated Problem. International Journal of Antimicrobial Agents, 34, 38-42.
- Clinical Review Safety Peripheral Intravenous Cannula; SPIVC Report. Clinical Evaluation Team, NHS. 161018.docx.
- Waitt C, Waitt P, Pirmohamed M: Intravenous therapy. Postgraduate Medical Journal
2004, 80(939):1-6. - Alexandrou E, Ray-Barruel G, Carr PJ, Frost SA, Inwood S, Higgins N, Lin F, Alberto L, Mermel L, Rickard CM; OMG Study Group. Use of Short Peripheral Intravenous Catheters: Characteristics, Management, and Outcomes Worldwide. J Hosp Med. 2018 May 30;13(5). doi: 10.12788/jhm.3039. PMID: 29813140.
- Rickard, C. M., McCann, D., Munnings, J., & McGrail, M. R. (2010). Routine resite of peripheral intravenous devices every 3 days did not reduce complications compared with clinically indicated resite: A randomised controlled trial. BMC Medicine, 8.
- Bolton, D. (2010). Improving peripheral cannulation practice at an NHS Trust. British Journal of Nursing, 19(21), 1346-1350.
- Carr PJ, Rippey JCR, Cooke ML, Higgins NS, Trevenen M, Foale A, Rickard CM. From insertion to removal: A multicenter survival analysis of an admitted cohort with peripheral intravenous catheters inserted in the emergency department. Infect Control Hosp Epidemiol. 2018 Oct;39(10):1216-1221. doi: 10.1017/ice.2018.190. Epub 2018 Sep 10. PMID: 30196798.
- Rickard CM, Webster J, Wallis MC, Marsh N, McGrail MR, French V, Foster L, Gallagher P, Gowardman JR, Zhang L, McClymont A, Whitby M. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012 Sep 22;380(9847):1066-74. doi: 10.1016/S0140-6736(12)61082-4. PMID: 22998716.
- Helm RE, Klausner JD, Klemperer JD, Flint LM, Huang E. Accepted but unacceptable: peripheral IV catheter failure. J Infus Nurs. 2015 May-Jun;38(3):189-203. doi: 10.1097/NAN.0000000000000100. PMID: 25871866.
- Wallis MC, McGrail M, Webster J, Marsh N, Gowardman J, Playford EG, Rickard CM. Risk factors for peripheral intravenous catheter failure: a multivariate analysis of data from a randomized controlled trial. Infect Control Hosp Epidemiol. 2014 Jan;35(1):63-8. doi: 10.1086/674398. Epub 2013 Dec 2. PMID: 24334800.
- Mangar D, Thrush DN, Connell GR, Downs JB. Direct or modified Seldinger guide wire-directed technique for arterial catheter insertion. Anesth Analg. 1993 Apr;76(4):714-7. PMID: 8466006.
- van Loon FH, Leggett T, Bouwman AR, Dierick-van Daele AT. Cost-utilization of peripheral intravenous cannulation in hospitalized adults: An observational study. J Vasc Access. 2020 Sep;21(5):687-693. doi: 10.1177/1129729820901653. Epub 2020 Jan 23. PMID: 31969049.
- van Loon FHJ, van Hooff LWE, de Boer HD, Koopman SSHA, Buise MP, Korsten HHM, Dierick-van Daele ATM, Bouwman ARA. The Modified A-DIVA Scale as a Predictive Tool for Prospective Identification of Adult Patients at Risk of a Difficult Intravenous Access: A Multicenter Validation Study. J Clin Med. 2019 Jan 26;8(2):144. doi: 10.3390/jcm8020144. PMID: 30691137; PMCID: PMC6406455.
Useful Tools
Learn More
If you would like to speak to a Vygon representative regarding how our IV Access Devices can support your Trust or to order a Smartmidline sample, please complete the form below.