Vygon (UK) Ltd announces that Bionector can now be used for up to 360 accesses
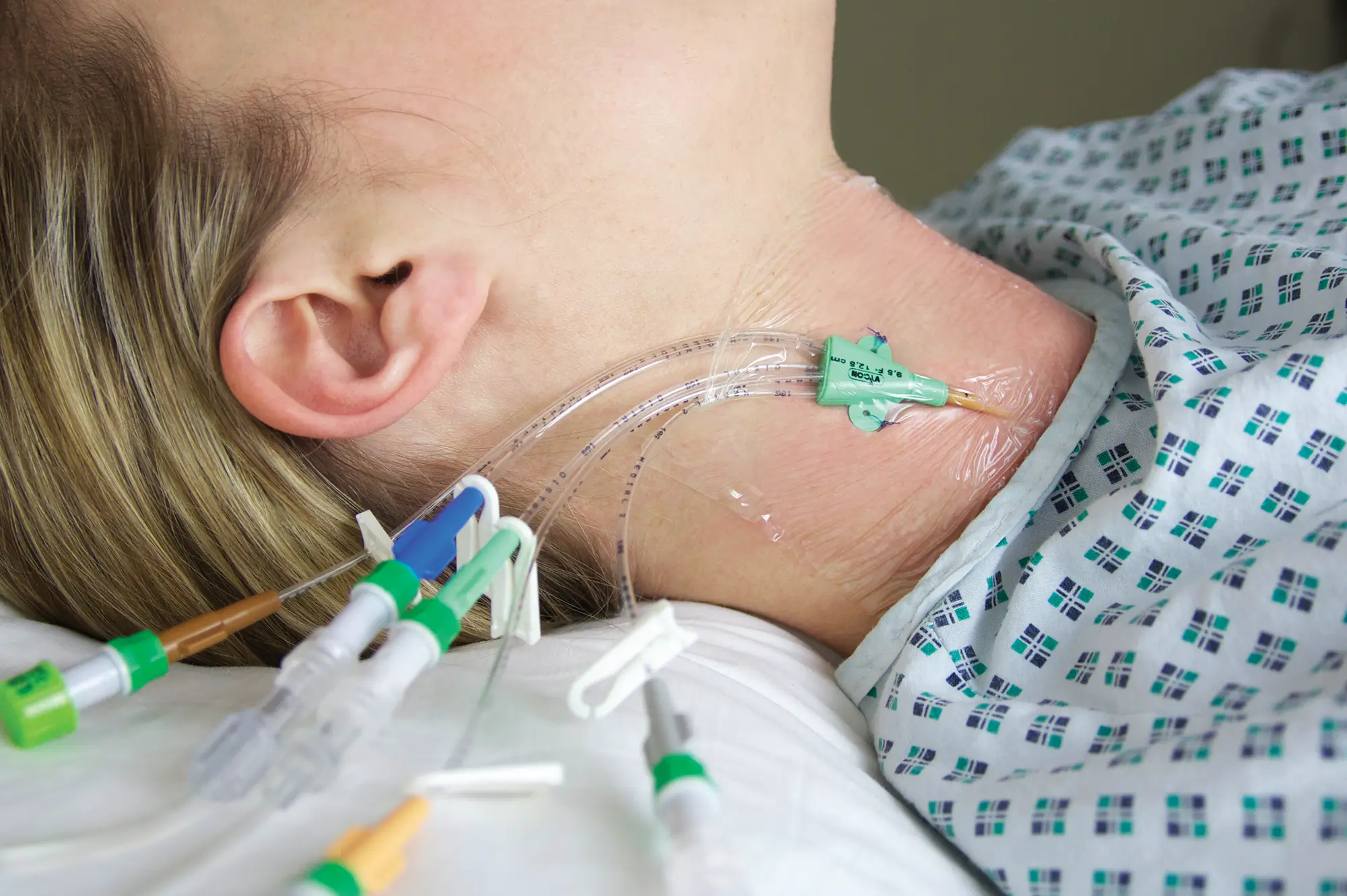
Vygon (UK) Ltd is pleased to announce an update to the instructions for use for Bionector – the closed, needle-free IV access system. The update means that Bionector can now be used for a period of seven days or 360 accesses, rather than seven days or 150 accesses as it was previously.
John Kerridge, Business Development Manager Vygon (UK) Ltd comments: The increase in the number of accesses of Bionector is fantastic for our customers as it offers them greater flexibility in their use of Bionector. It extends the use of their product which in turn can provide them with cost savings and greater efficiencies. There is currently an awful lot happening in the needle-free market and Vygon is putting a lot of time and effort into the future development of Bionector watch this space!