Reducing Complication Risks with Implantable Ports

As with all procedures, reducing complication and infection risks should be a high priority for all healthcare providers. With some studies suggesting that healthcare-associated infections cost the NHS an estimated £2.1 billion per annum. (Guest JF, Keating T, Gould D, 2020).
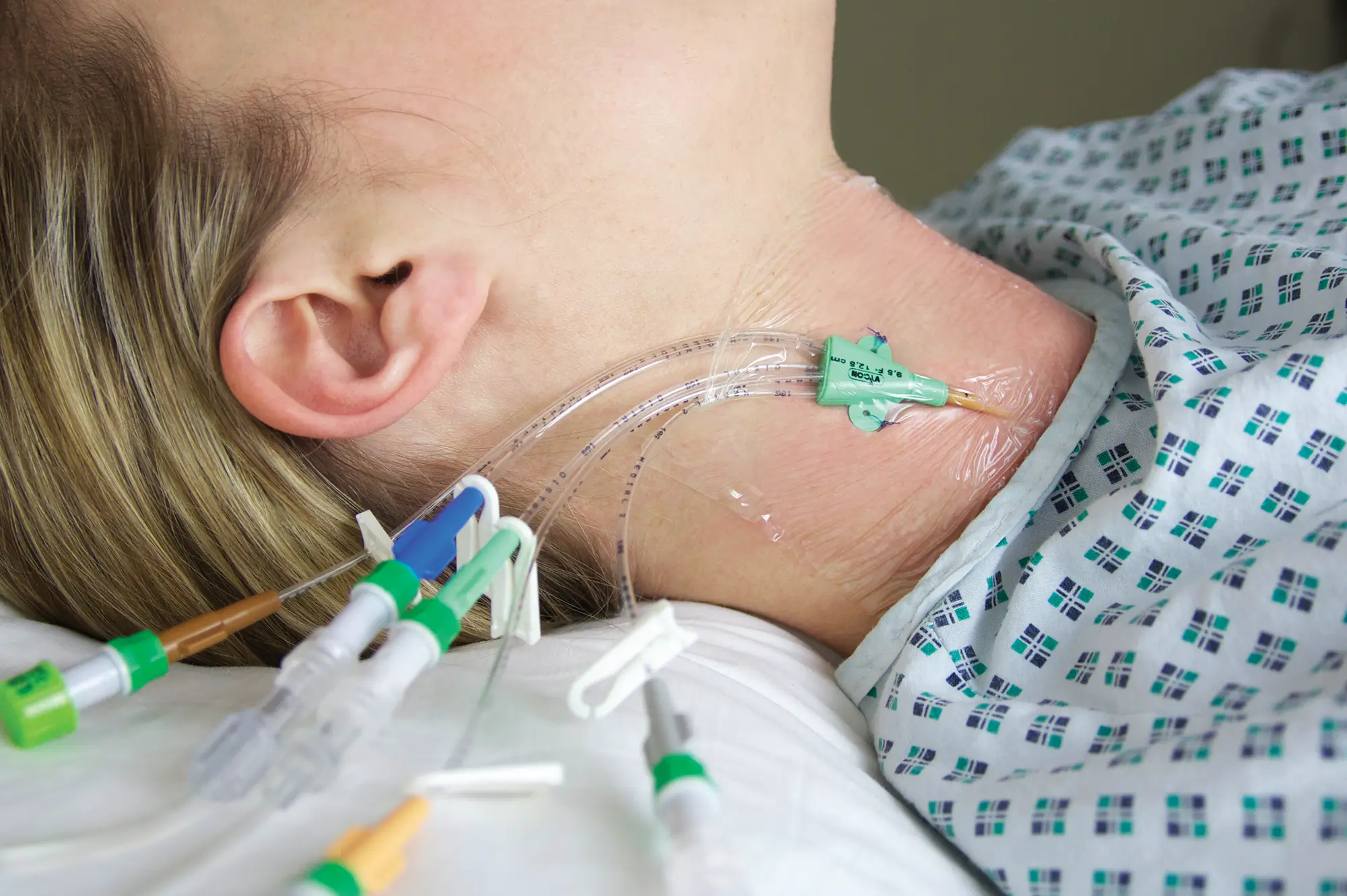
This is of particular importance when encountering a vascular access device, as direct access to the patient blood stream increases risk of complications – especially in immunocompromised patients such as those with cancer.
In this article we explore the possible complications associated with a totally implanted vascular access device (TIVAD), and the considerations and steps that can be undertaken to help mitigate these risks.
Complications Associated With Totally Implanted Vascular Access Devices
Early Complications:
- Malposition: Intravenous, cardiac
- Arrhythmia
- Perforation and bleeding
- Arterial malpositioning
- Pneumothorax
- Thoracic duct injury
- Air embolism
Delayed Complications:
- Infection
- Venous thrombosis, pulmonary embolism
- Venous stenosis
- Catheter pinch-off, fracture and migration
- Catheter embolisation
- Air Embolism
Initial Selection of Venous Access Devices
One of the most important steps a clinician takes, is upon the initial selection of which vascular access device a patient will have placed for the duration of their treatment. Selecting the right device for each patient can impact the risks of complications during therapy.
With many options available, the choice should be made on number of variables including:
- Type of vascular access required (central or peripheral)
- Types of therapies the device will be used for
- Solutions required for therapies (viscosity of solutions and irritant/non-irritant solutions)
- Expected duration of therapy
- Potential complication risks
- Impact on patient well-being
- Patient preference (where applicable)
It’s important to note that at present the selection of vascular devices is not standardised across the UK and can vary between hospitals and trusts. This choice can be impacted by other external variables such as department resource, cost of device, and clinician preference.
Central Venous Access Devices for the Delivery of Systemic Anticancer Therapy (CAVA).

The UK trial (CAVA) found that among central venous access devices, totally implanted ports were associated with significantly reduced rates of complications, when compared tunnelled central venous catheters and peripherally inserted catheters (PICCs).
The study focused on the central venous delivery of systemic anti-cancer therapy, with the primary outcome measure of complication rate. Complication rate included; infection, venous thrombosis, pulmonary embolus, inability to aspirate blood, mechanical failure and other factors.
The study concluded ‘For most patients receiving [systemic anticancer therapy], PORTs are more effective and safer than both Hickman and PICCs. Our findings suggest that most patients receiving [systemic anticancer therapy] for solid tumours should receive a PORT within the UK National Health Service.”
Although this study focused on vascular access device selection within the specifics of anti-cancer therapy, with these results a case could be made for increased port placement over other methods of vascular access in patient circumstances where appropriate.
Implantable Port Material Considerations – Plastic, Titanium or Hybrid Ports
If a TIVAD (port) has been chosen as the selected device for vascular access, considerations should also be given to the type of port selected prior to insertion. Recent studies have suggested that certain types of materials for ports can increase the risk of particulate build up when in situ.
A Vygon study identified a link between the types of port materials and punctures by huber needles, and the rates of particulate matter build up.
As can be seen in the magnification images above, ports with plastic reservoir (right) can receive significant surface impact damage from huber needle placement. It is worth noting that the compared hybrid ports (with titanium reservoir) received far less surface damage during use.
The study by VIN demonstrated that the port surface damage could lead to opportunities for material build up in these areas. As with any material build up in vascular access sites, this could lead to complications such as: embolism by mechanical blockage, inflammation, microthrombi formation, potential to cause phagocytic overload and the potential result of secondary infections.
The results of this study demonstrated that mean quantity of particulate matters were 5.2 times higher in plastic ports than with hybrid ports with a titanium reservoir.
Implanted Port Size Selection
If a TIVAD (port) has been chosen as the selected device for vascular access, considerations should also be given to the type of port selected prior to insertion. Recent studies have suggested that certain types of materials for ports can increase the risk of particulate build up when in situ.
Prior to insertion, consideration should be given to the correct size port for the patient. Selecting the right size port for the patient can aid in the prevention of such complications as: malpositioning, perforation, patient discomfort, and device failures/breakages.
Clinicians should take the following into account when selecting an implanted port device:
1) The patients age (paediatric or adult)
2) The patient’s size (is the patient over or underweight, or above/below the size for their age)
3) Choice of port placement site (where the port is to be placed on the chest, or arm)
The clinician placing the device will want to ensure that the port is placed in a pocket that is not too big or too small to achieve optimum outcomes.
Standardisation of Maintenance Practices of Implanted Ports for Clinicians
Some of the most effective infection prevention measures are the tried and tested methods, of which all clinicians should be aware of. When encountering an implanted port (or any vascular access device), clinicians should consistently practice the following:
- Sterilisation of the area and insertion devices
- Thorough handwashing technique
- Dressing ports post insertion and after subsequent access
- Consider therapeutic use to reduce number of needlestick entries
- Regular flushing
- Site monitoring
Patient education on Implanted Port Care
As many port patients can be treated in outpatient settings and have the device in-situ when at home, a case can be made for teaching patients to care for their port site.
To reduce risks of further complications patients should be educated on:
- Education on hygiene procedures when in contact with, or around the port site
- Education on site monitoring for early complication intervention
Developing and offering a ports service for patients is a great addition for any trust. But as with any vascular device mitigation, and reduction of possible complications, should be at the forefront of every practice.
To ensure you get the best out of your service, Vygon is on hand to support you every step of the way. We not only offer a full range of vascular access devices, but are available to advise on clinical training and support, as well as patient guidance.
To discuss your departments requirements, use the button below to get in touch.