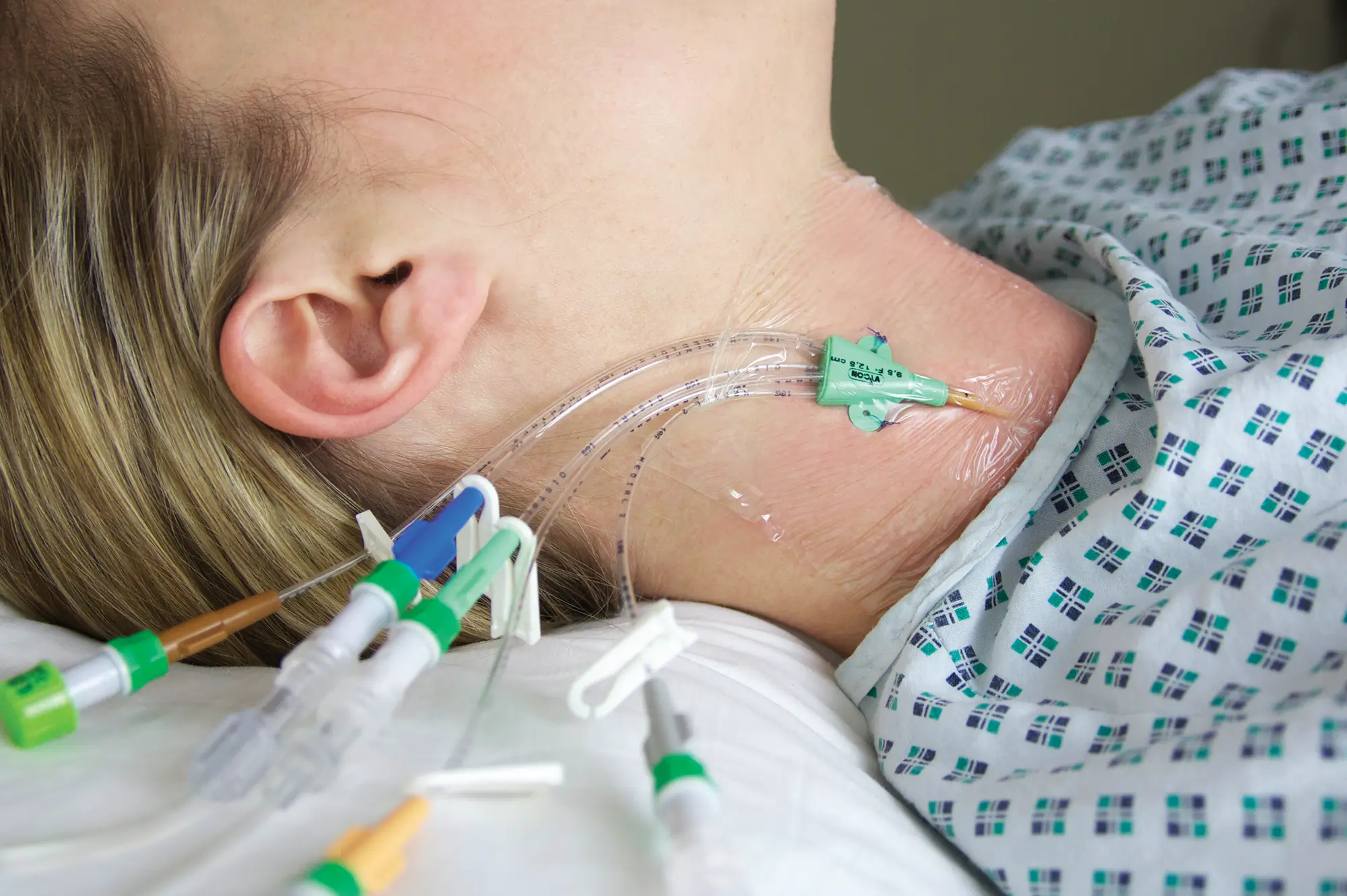
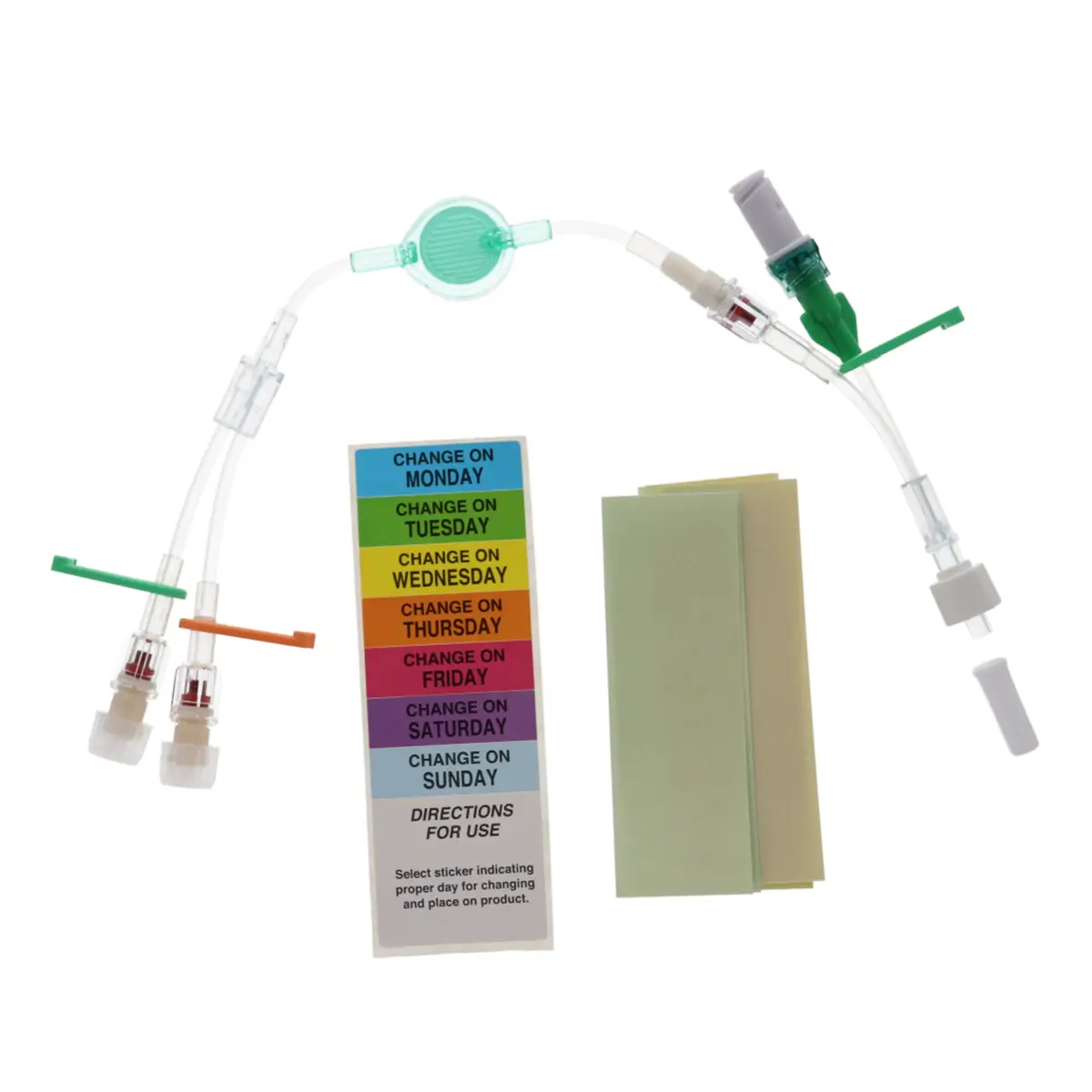
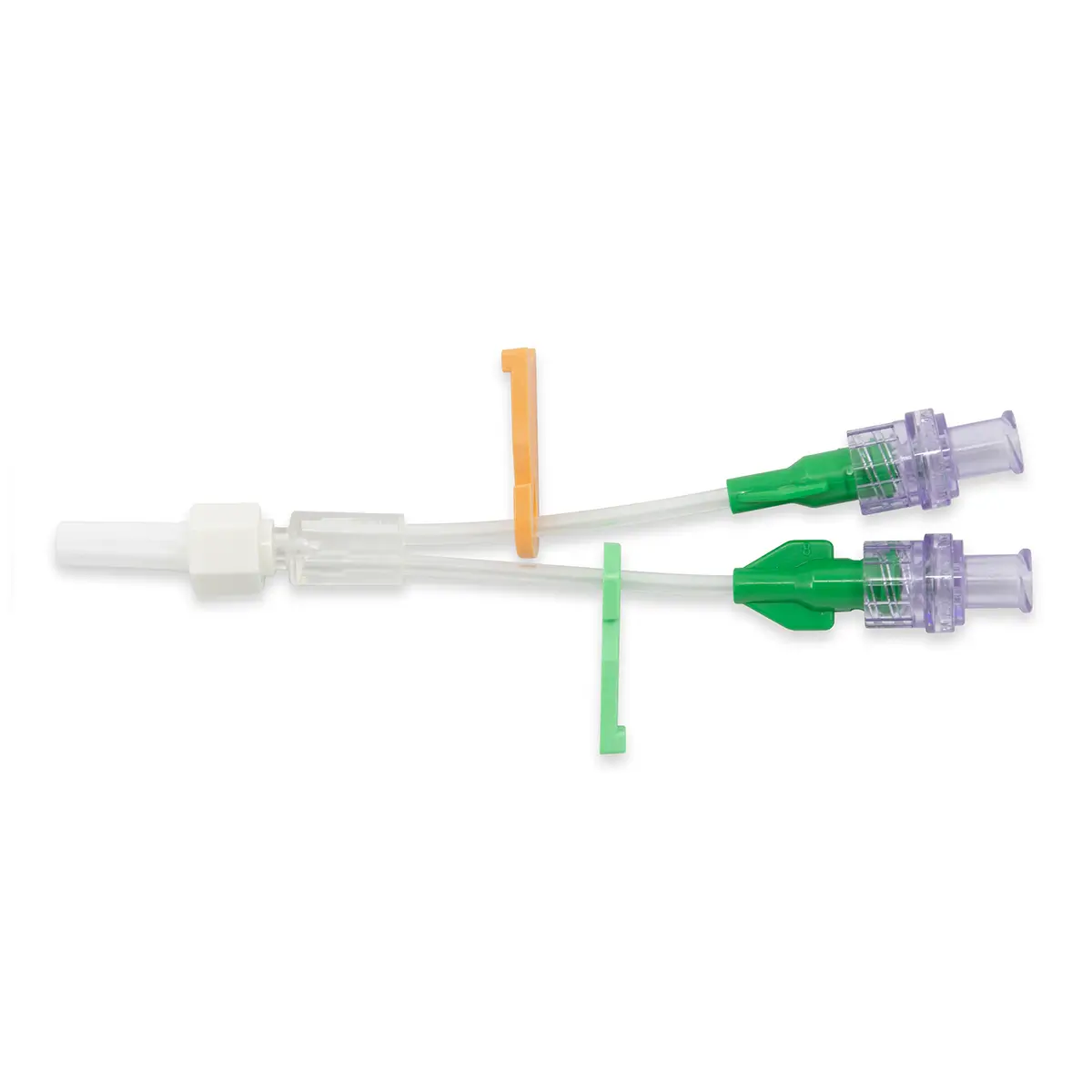
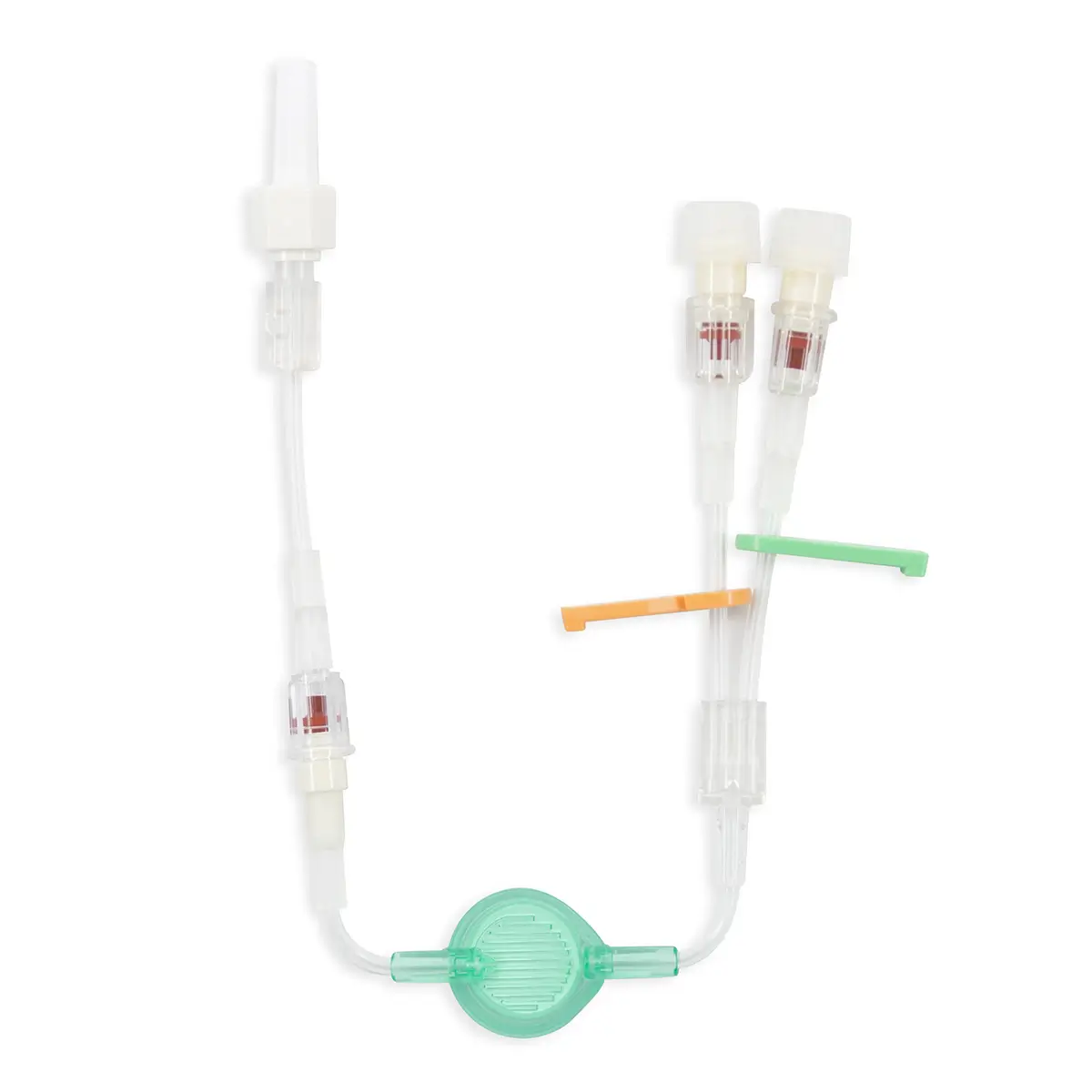
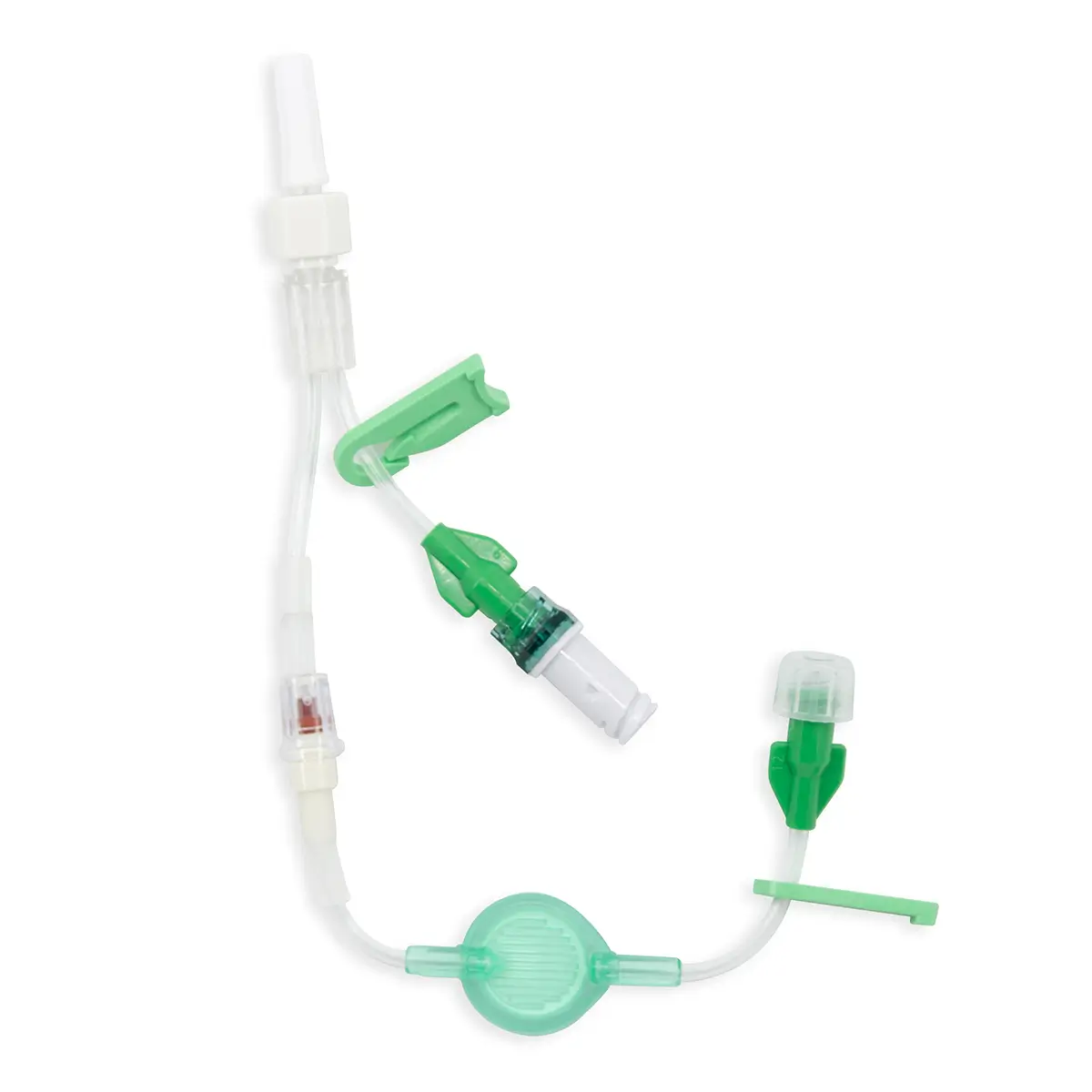
The Ready-Set range is a range of modular I.V. accessories specially designed for neonates and infants. It allows the practitioner to select the most suitable I.V. administration system for these patients.
Benefits of the device:
– Removes the need for additional 3-way taps, ramps and extension lines therefore reducing the risks associated with multiple connections such as air embolism, blood loss…
– Allows the filtration of the infusions thus reducing particules contamination resulting from the mixing of drugs, solutions etc …
– The closed-system needleless connectors (Bionector) fitted on some of the lines allow to reduce the risk of infection.
– Non-return valves avoid backtracking of the infusions in the other lines.
Home / Neonatology and Paediatrics / Neonatal Catheters, Introducers and Cannulas / Ready Set Unite
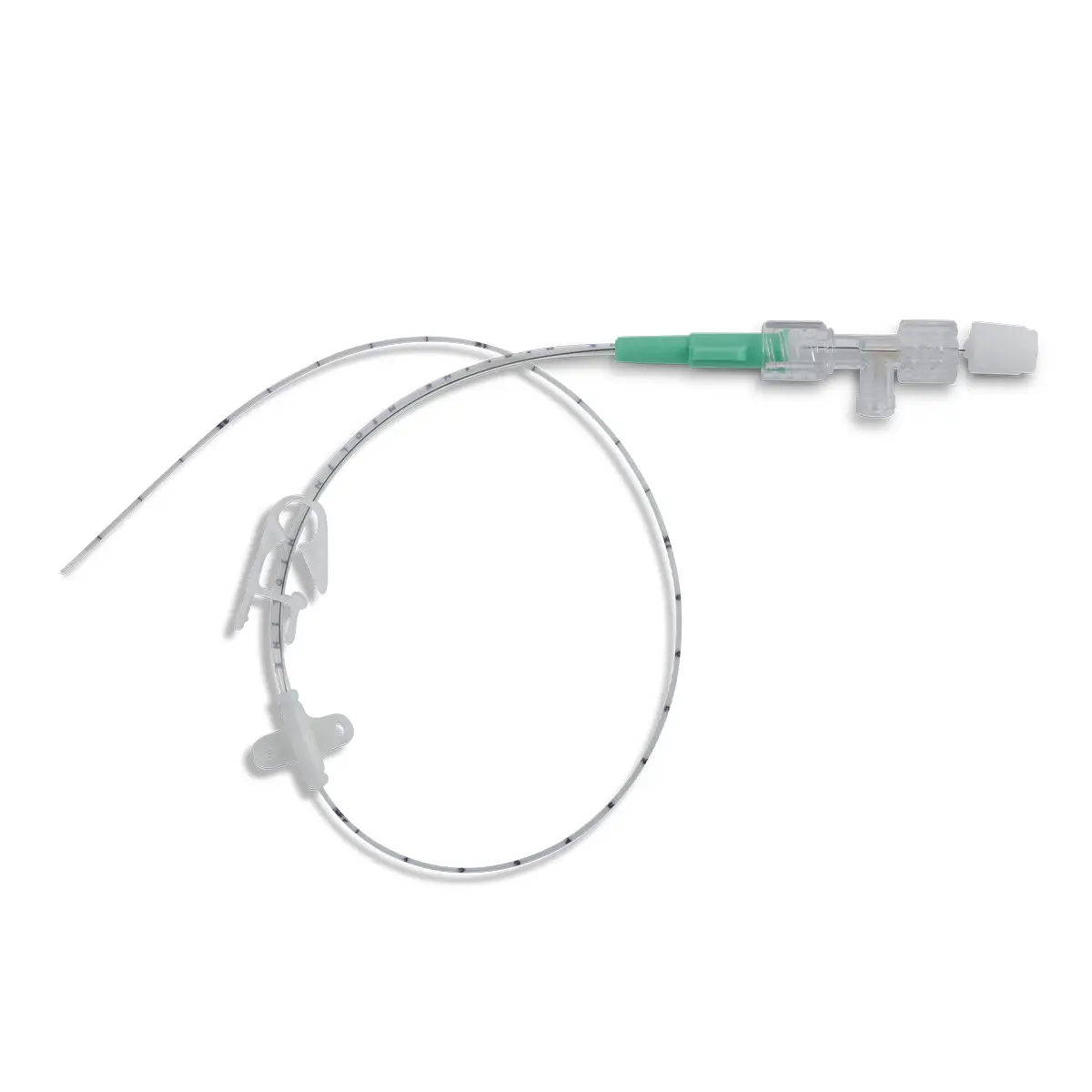
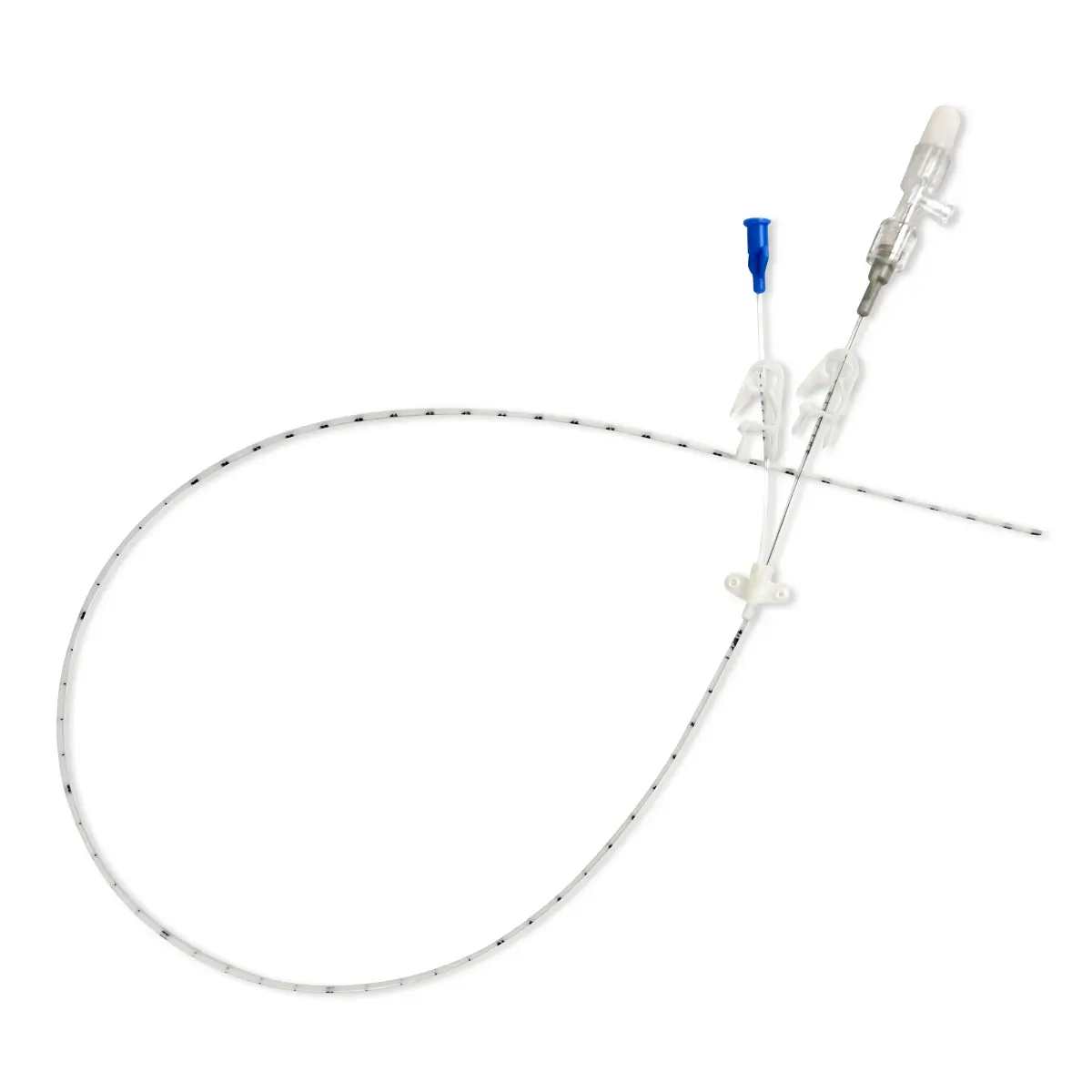
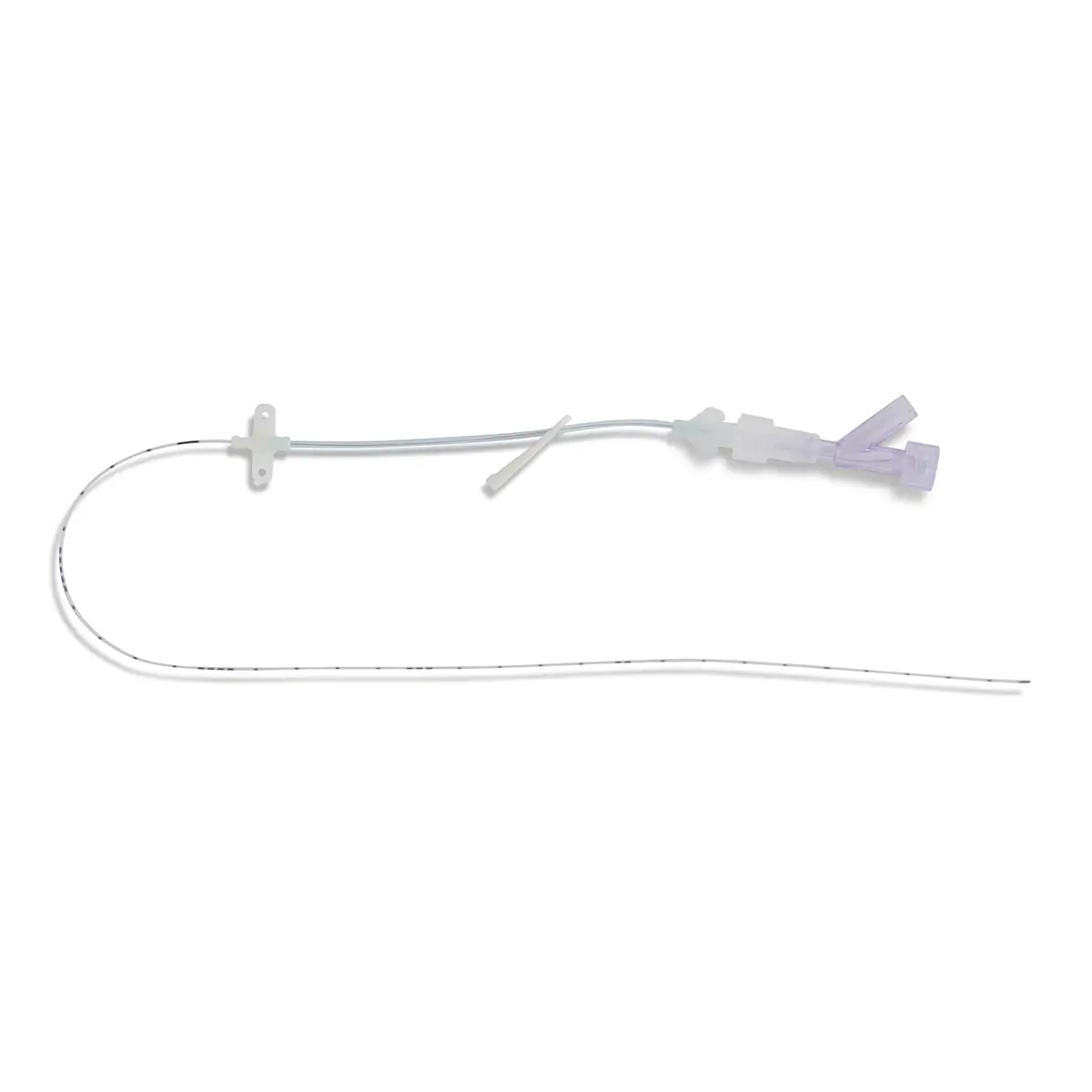
Code 836.205: Ready-Set “Unite”
Polyurethane extension tube with 3 access ports. Total priming volume: 1.3 ml
Main line (20.5cm long) with 2 access ports incorporating non-return valves and clamps. This line is also fitted with a 0.22µm filter, 96 hours with low proteic adsorption membrane. This air-eliminating filter is not suitable for blood, blood products or lipids. Flow rate: 3.5 ml/min
The non-return valve on the distal part of the line avoids the occlusion of the filter by reflux of lipidic solutions if the other line is used for that purpose.
Lumen 2 (2cm long): this line incorporates a closed-system needleless connector (Bionector) and can be used either for intermittent injections of medication or for continuous infusions (e.g. lipidic solutions). Priming volume: 0.26ml – Flow rate: 90 ml/min
Supplied with labels showing the day that the line must be replaced and colour labels to indicate the type of solution or drug infused in each lumen.
UOI Units: Box
UOI: 10
Contains Latex: No
Contains DEHP: No
Pyrogen Free: Yes
Sterile Medical Device: Yes

New Patient Resources from our Partners at Avanos
Our partners at Avanos have recently launched tubefed.co.uk – a dedicated website platform that offers a comprehensive guide to tube feeding. …
Read More
Using the LISA Technique for Reducing Neonatal Intensive Care
Utilising the LISA Technique in a local neonatal unit, for reducing neonatal intensive care January 2022 Introducing the LISA technique …
Read More
Increasing delayed cord clamping in c-section births
Our Neohelp™ heat loss prevention suit supports neonatologists perform more delayed cord clamping in c-section births by keeping neonates at …
Read More