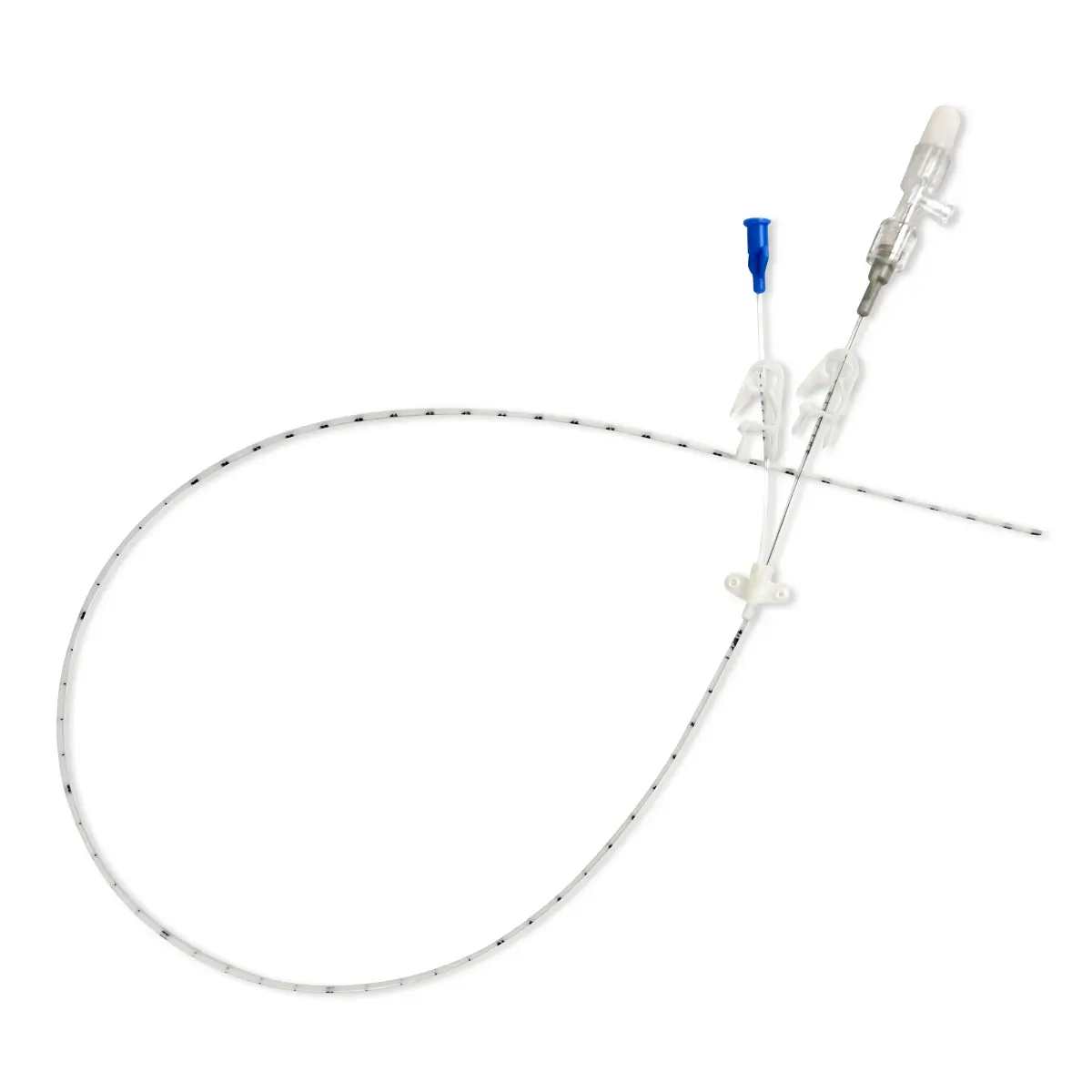
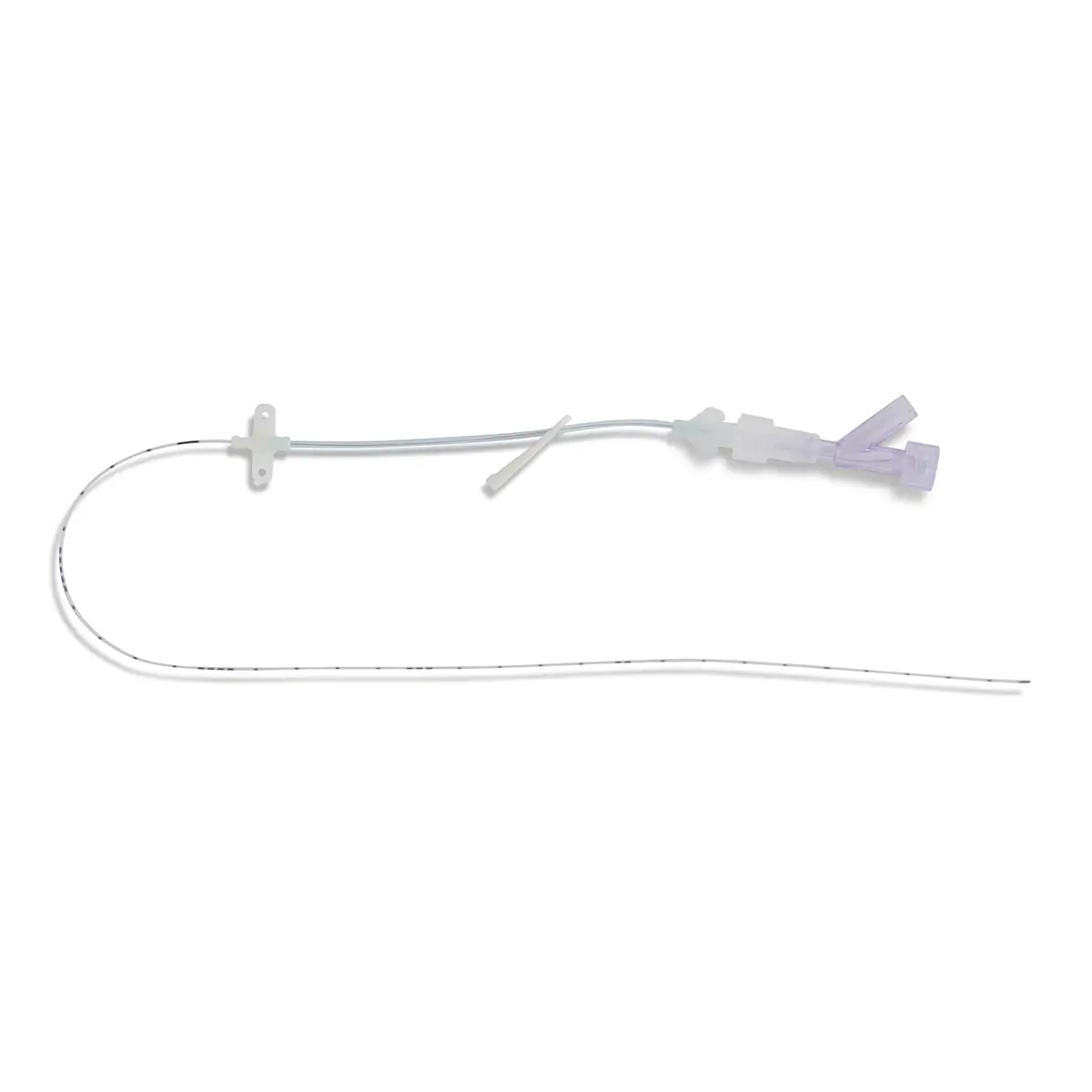
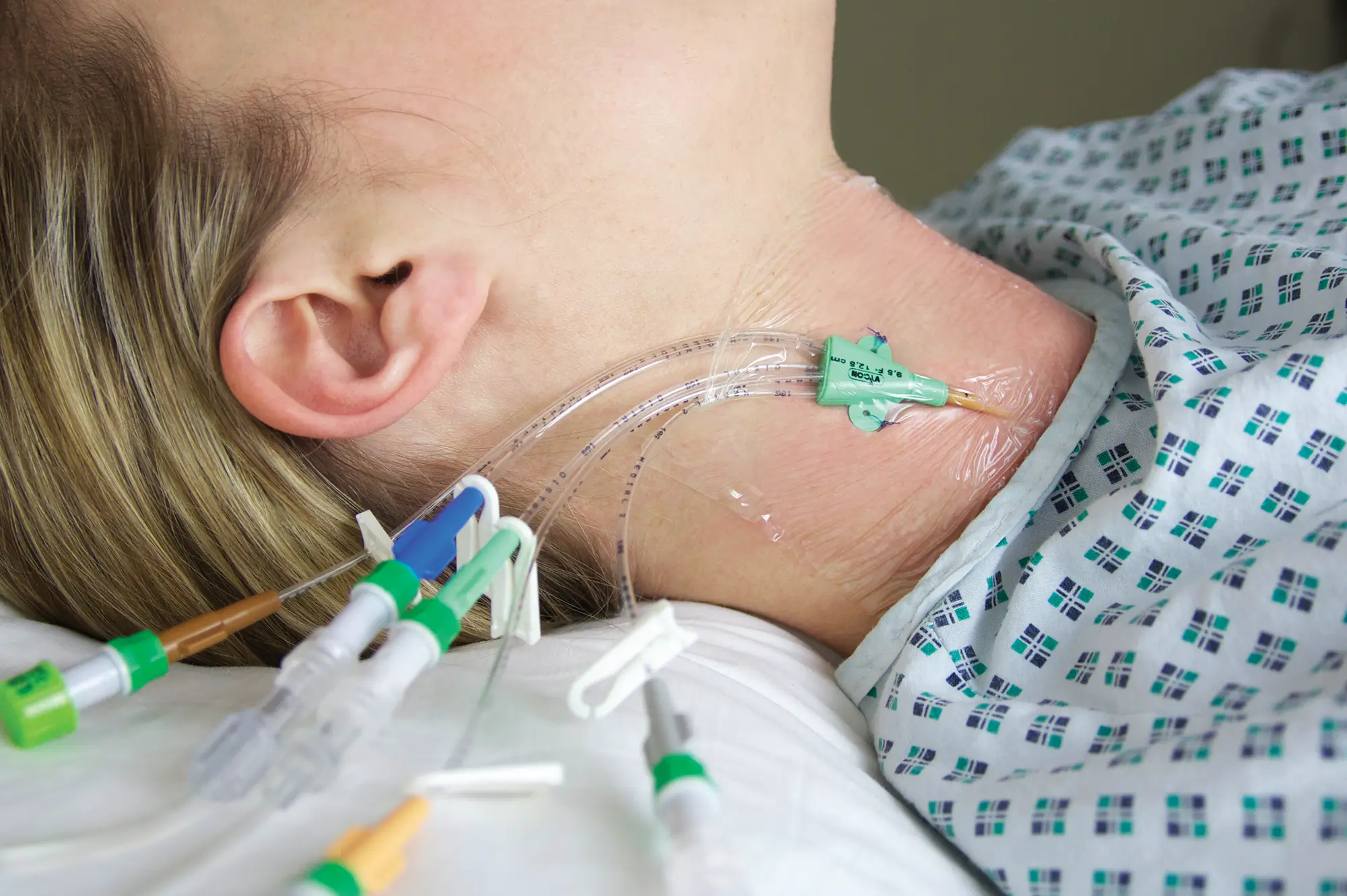
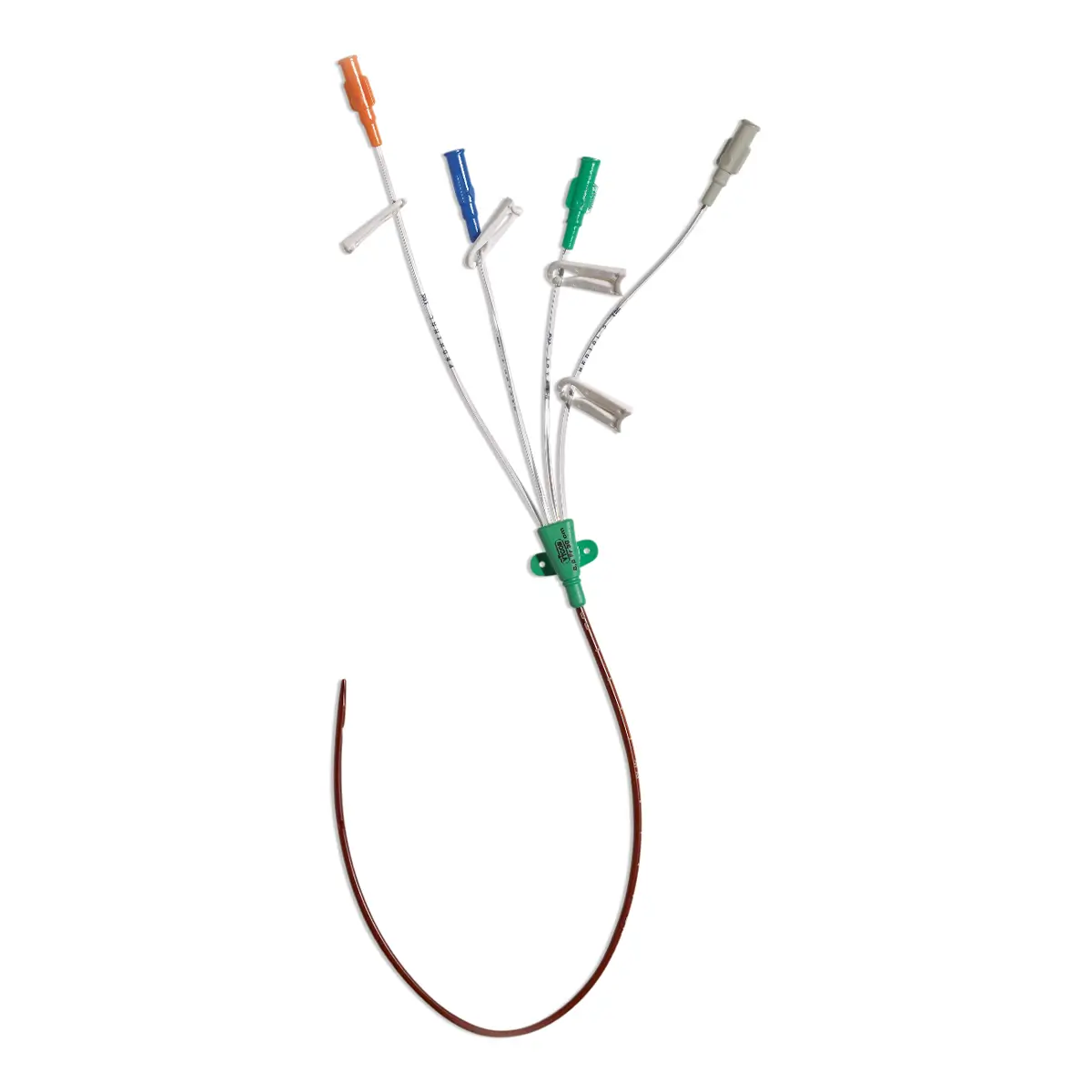
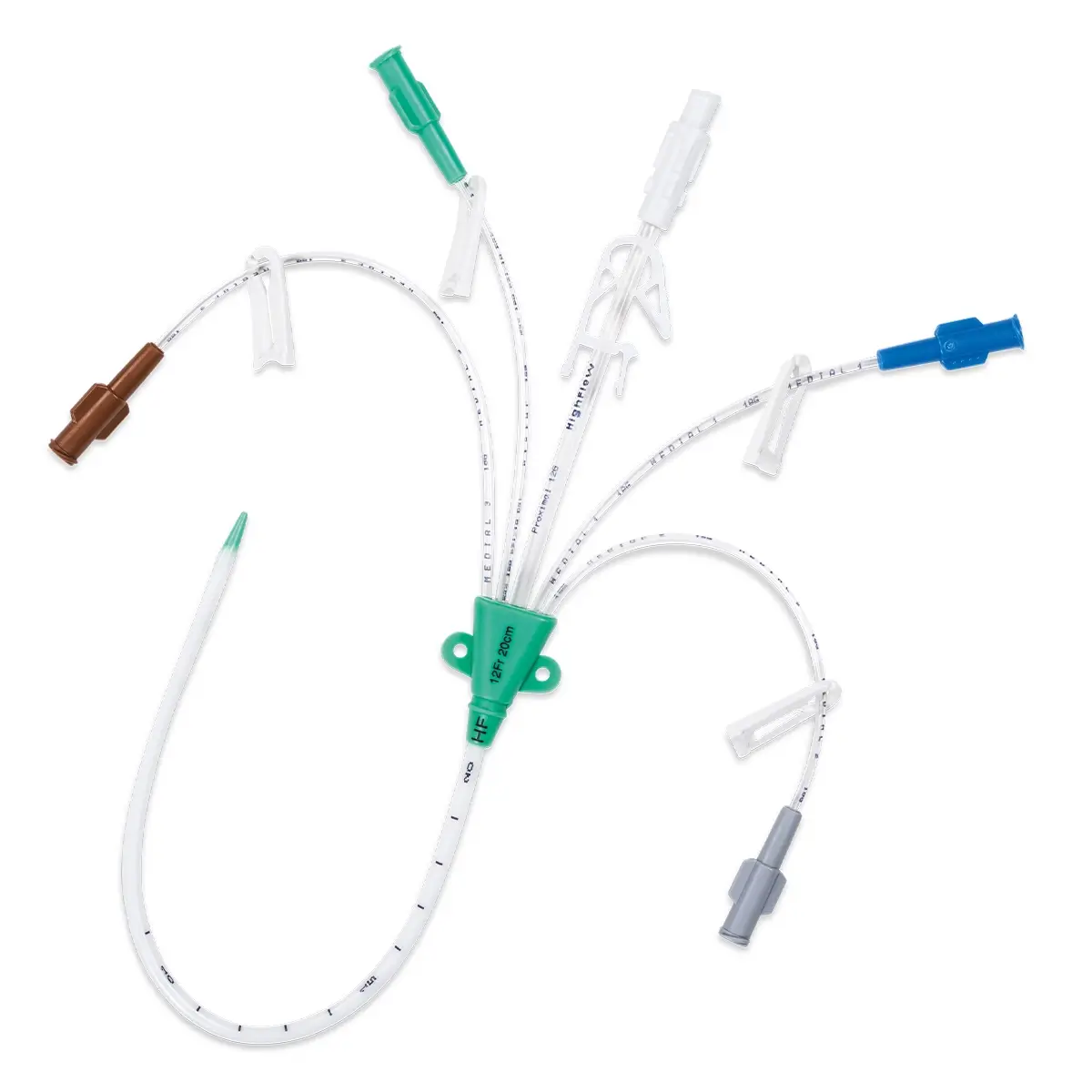
Antibiotic impregnated multi-lumen catheter.
Benefits:
– Biocompatible and safe
– Clinically proven to reduce CRI and colonisation
– Effective broad spectrum of activity against bacteria and fungi
– Long lasting: will protect for the life of the catheter
– Nitinol “J” guidewire for smooth catheter placement
– CE registered. CE 0481.
Tray presentation with label for traceability containing:
– 1 four lumen XRO catheter with extension tubes fitted with a clamp (length: 16 cm code 6158.167, 20 cm code 6158.207, 30 cm: code 6158.307)
– 1 puncture needle length 70 mm, 18G
– 1 short I.V. cannula length 70 mm, 18 G
– 1 graduated “J” guidewire and guidewire advancer, Ø 0.88 mm – Length 70 cm
– 1 dilator
– 1 syringe 5 ml
– 1scalpel
– 1 secondary fixation wing
– 4 injection caps
Multistar is available in 3 lumen: see corresponding technical files code 6155 and 5 lumen, see corresponding technical files code 6159.