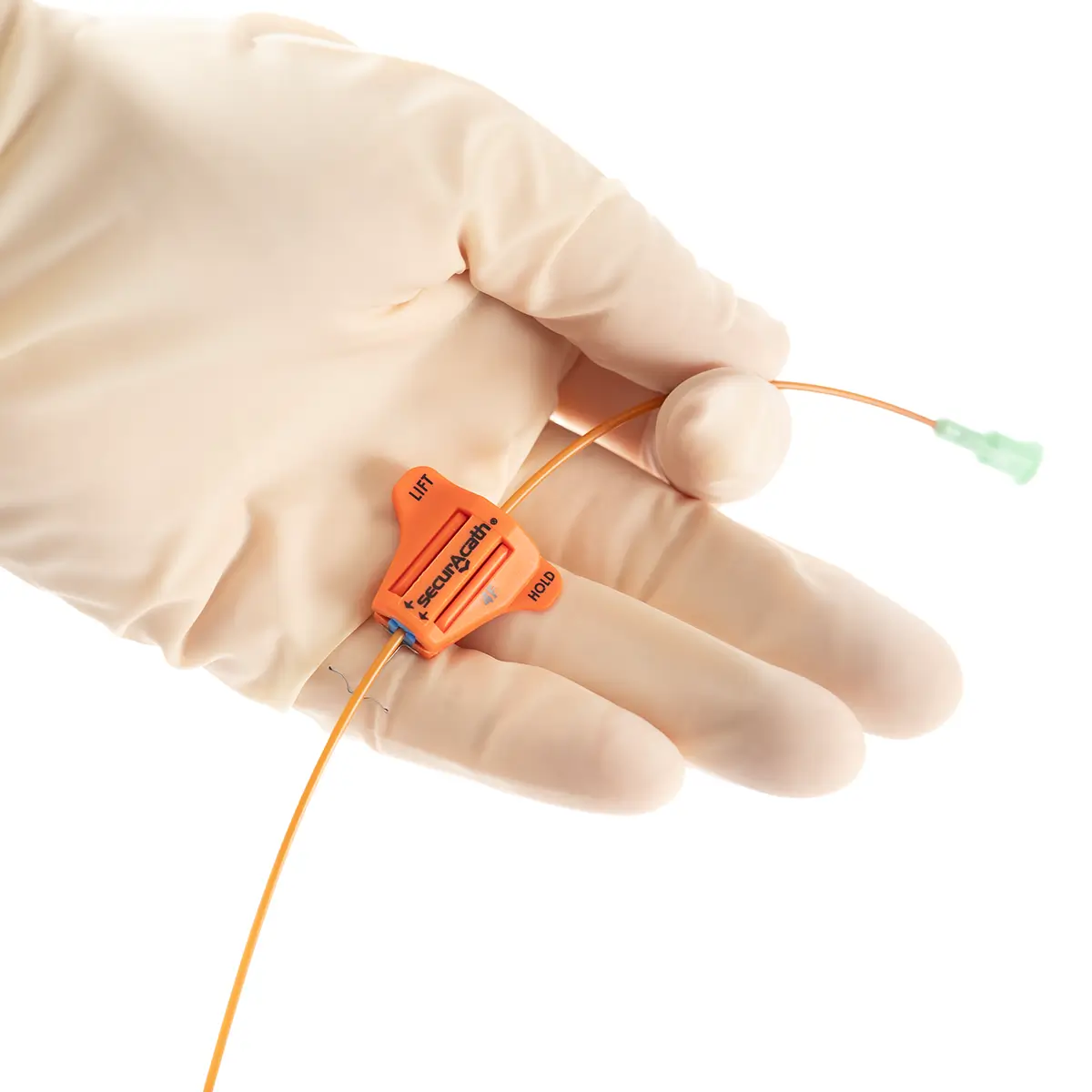
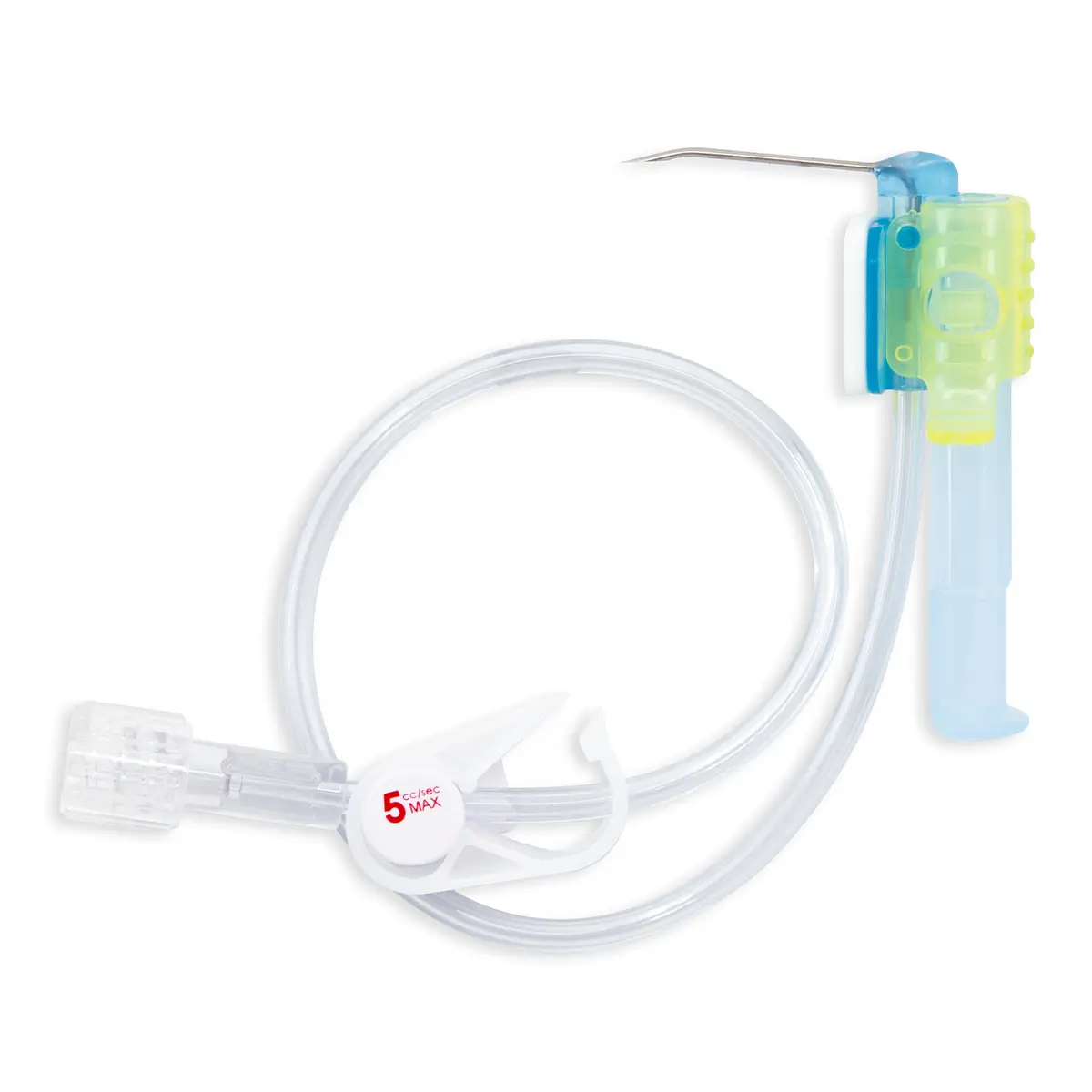
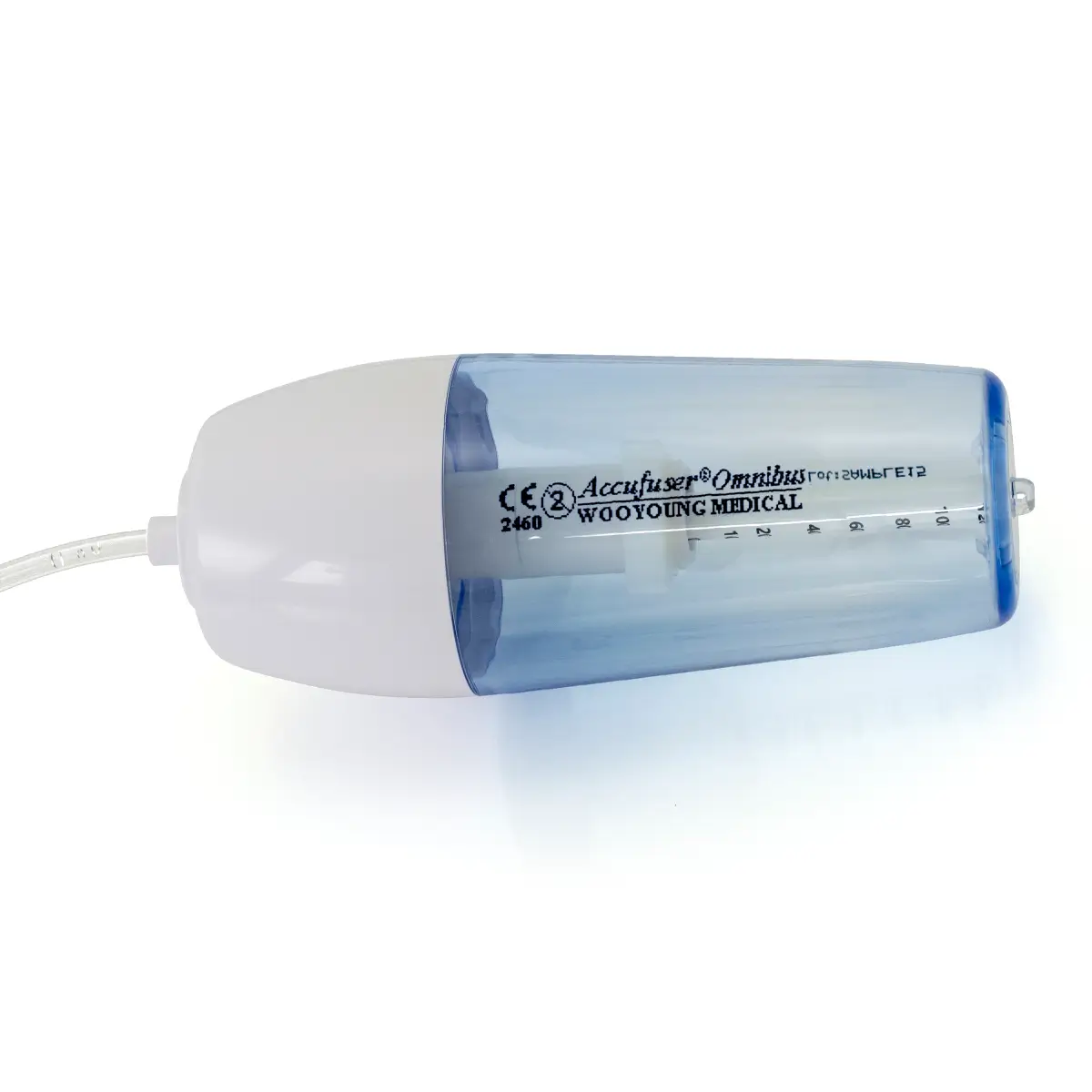
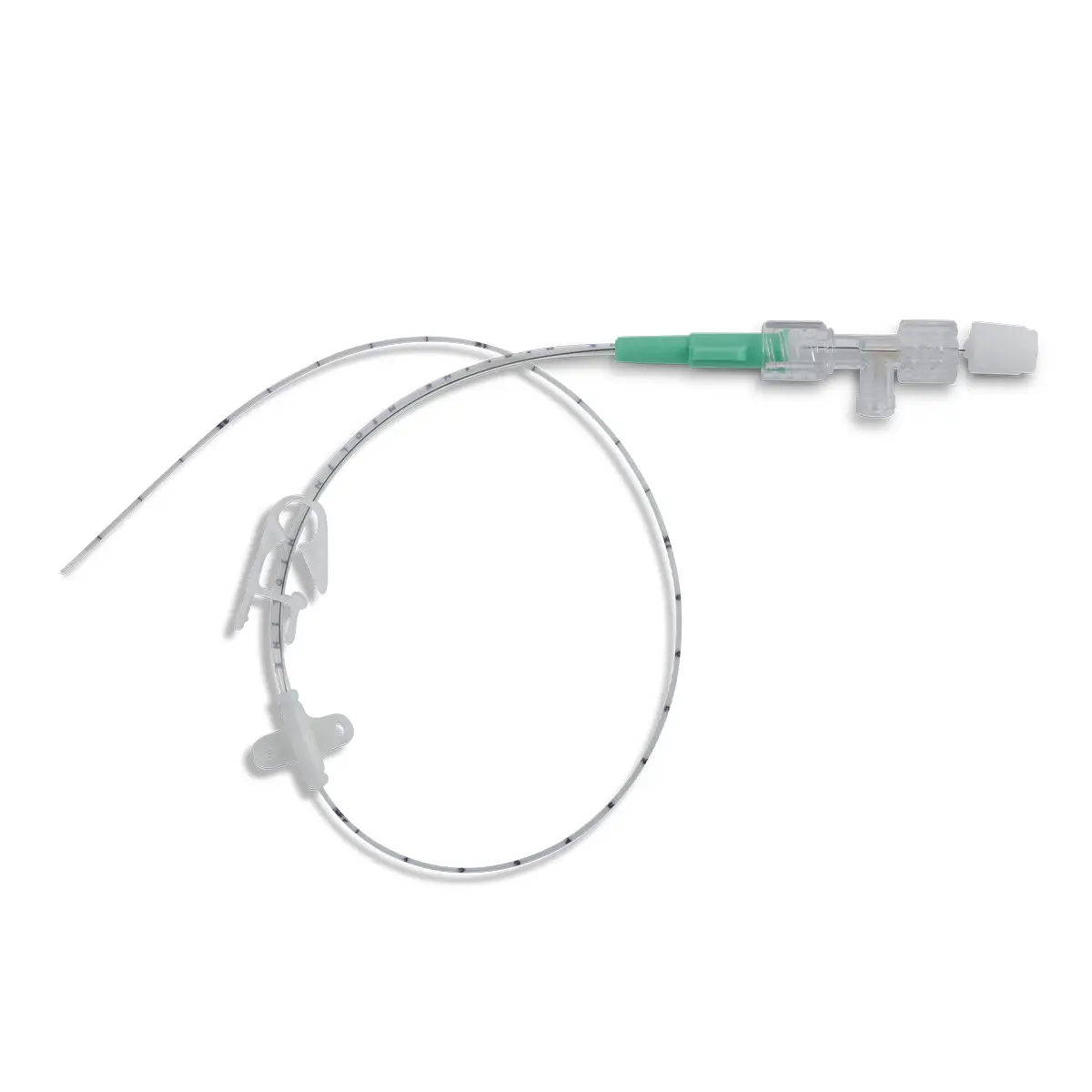
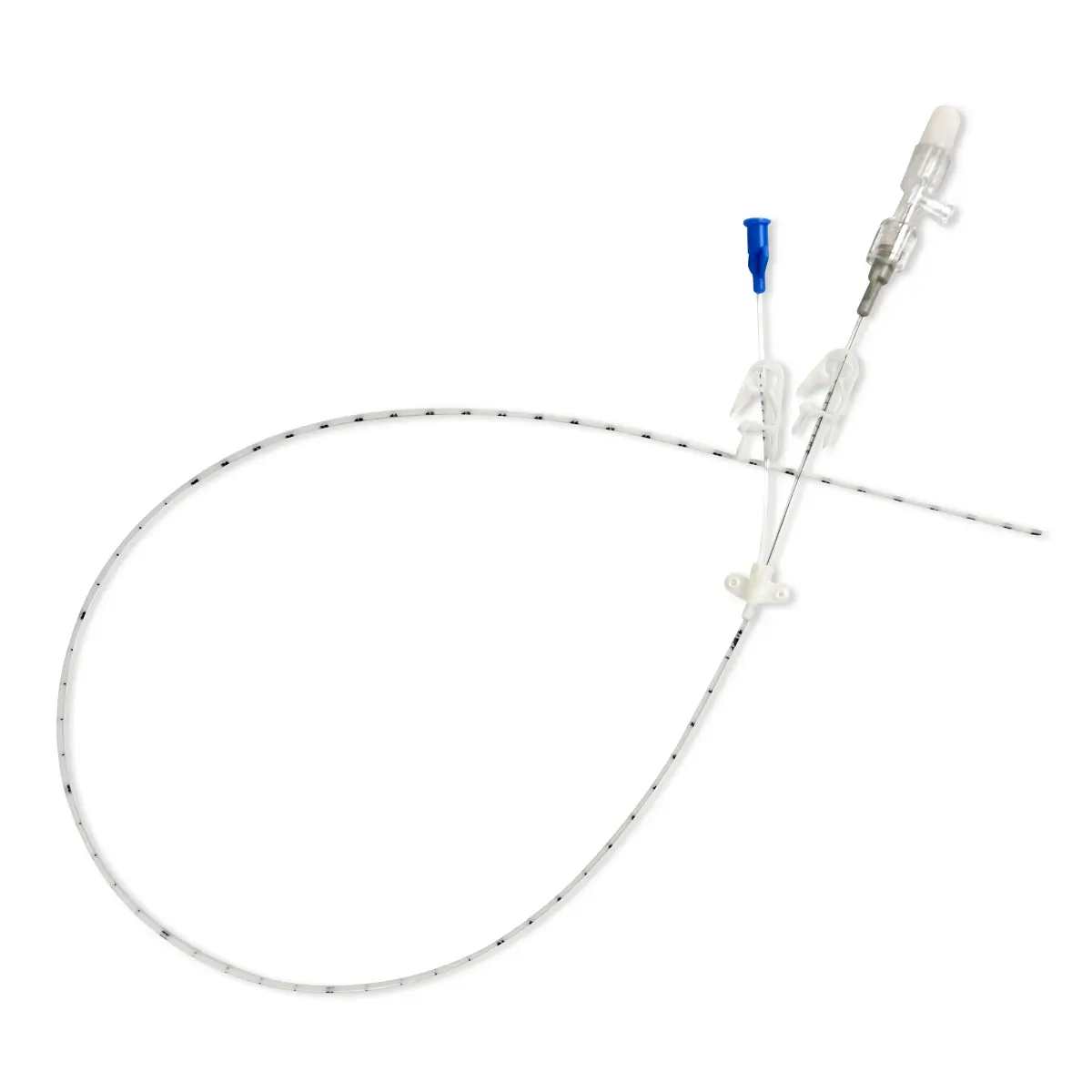
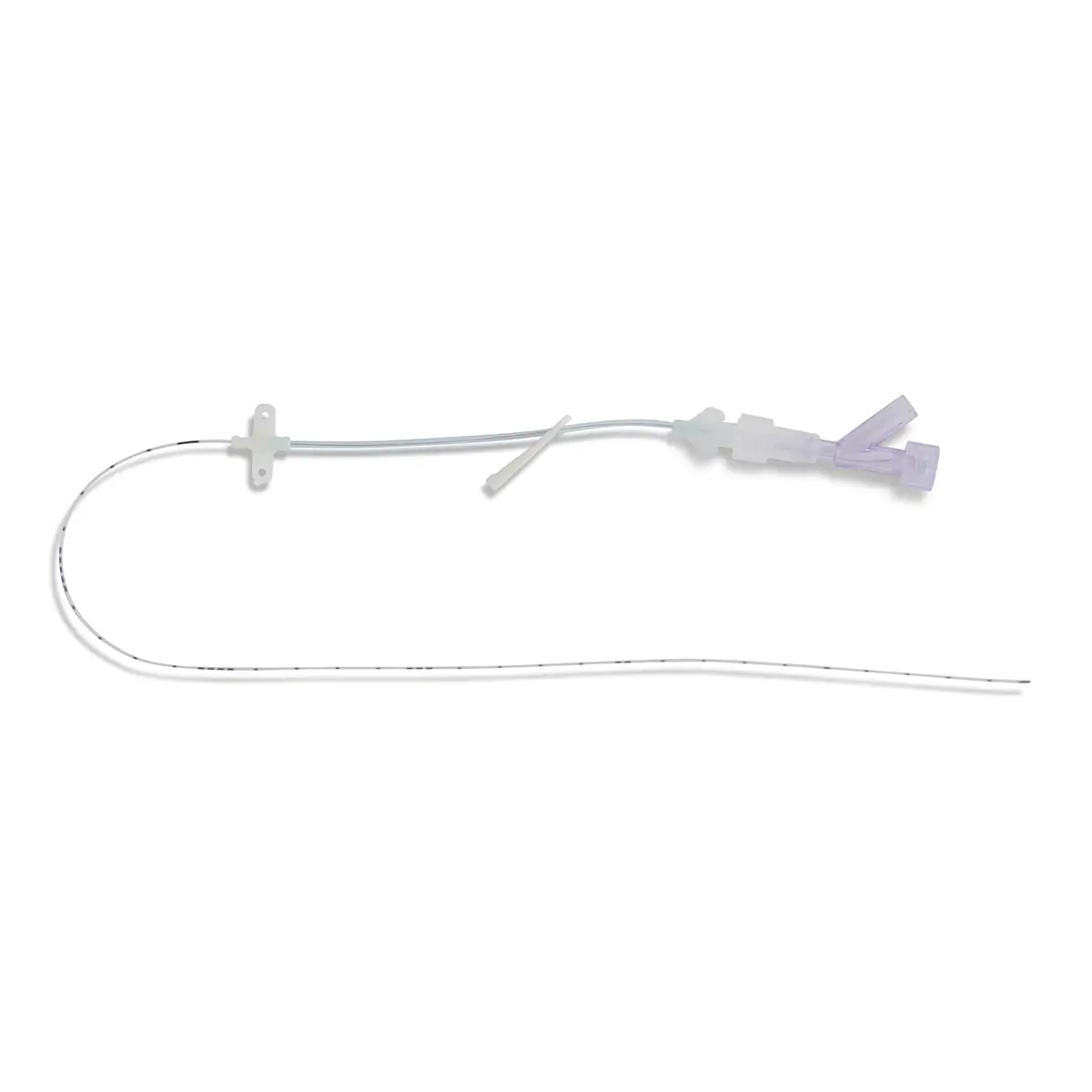
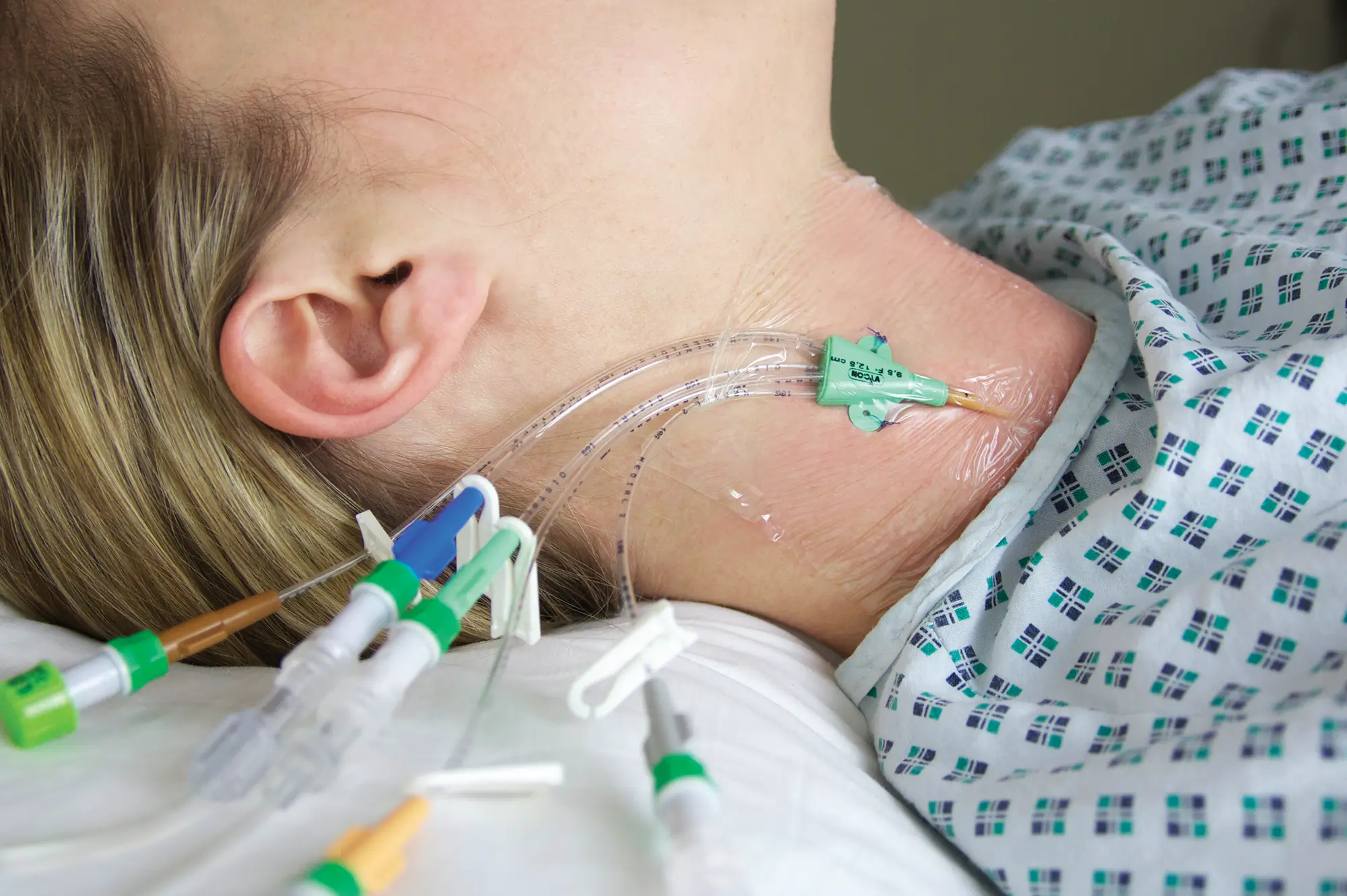
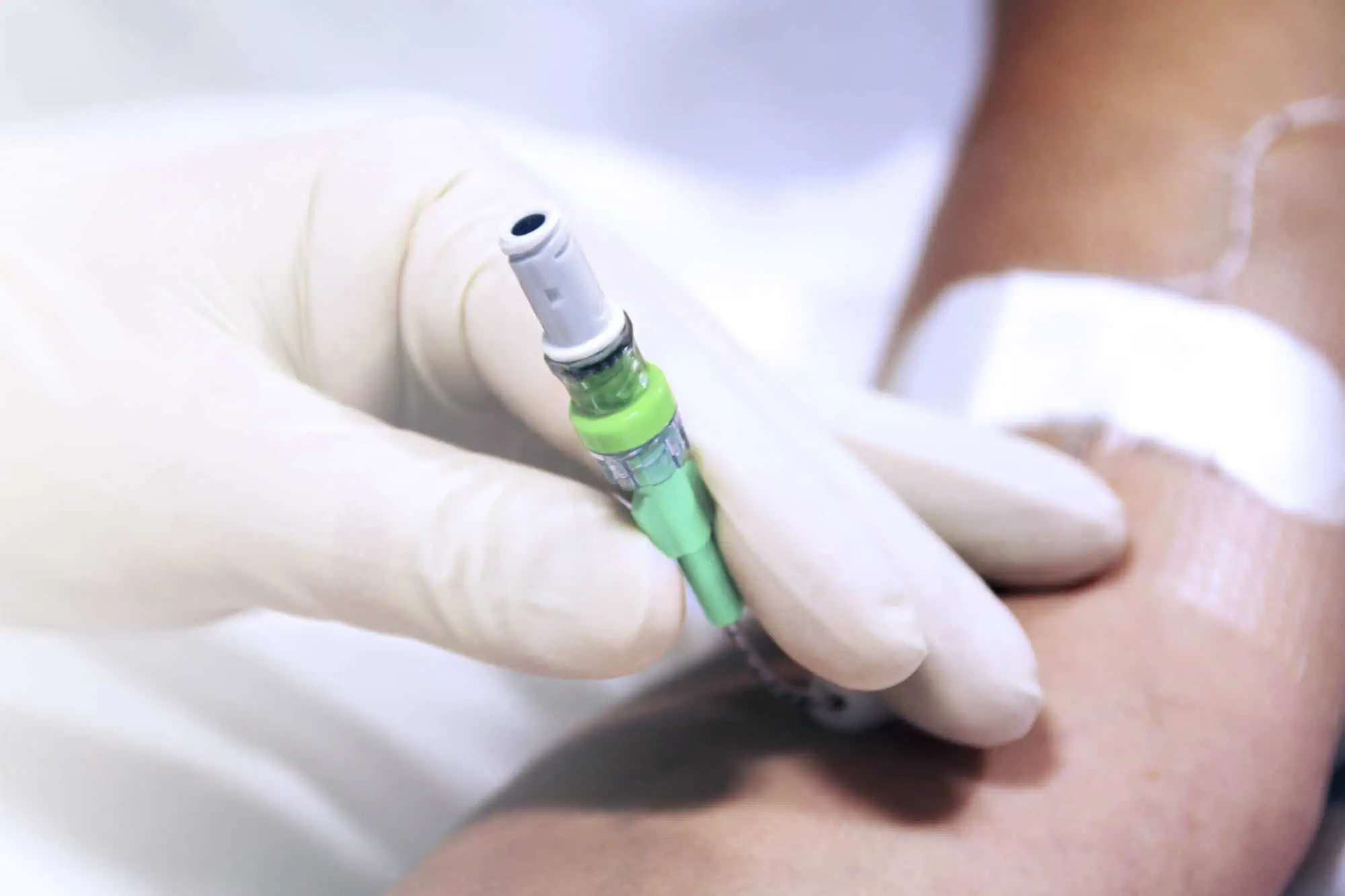
seesite™ is an easily administered implantable lightweight, hybrid port for paediatrics and brachial placement. It is positioned directly beneath the skin, usually in a discrete easily accessible place (chest level) with unique radiopaque marking with max flow rate identification. Medicines then reach the blood circulation directly after simply puncturing the site through the skin.
All seesite™ 2000 Series Micro Ports with an attachable 5Fr, 60cm silicone catheter and US-guided MST insertion kit including 5Fr introducer.
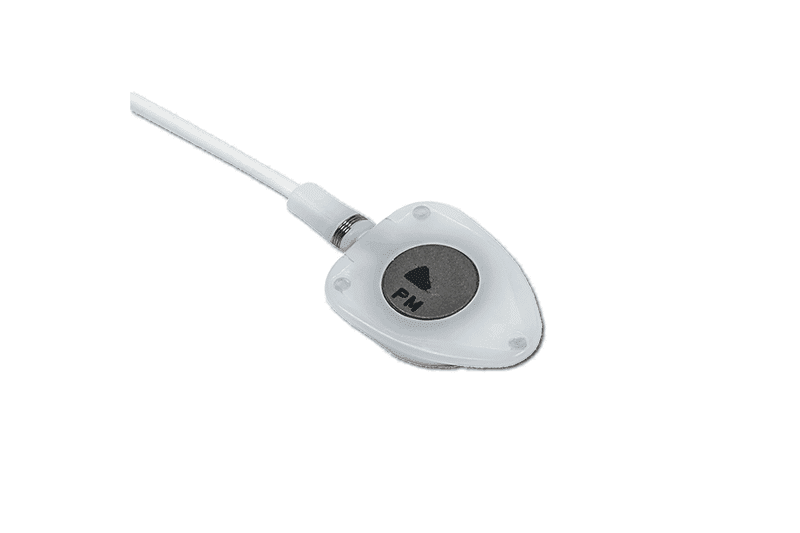
seesite™ unique and innovative radiopaque marking:
- Radiopaque marking detectable by X-ray
- Silicone filled suture holes
- Each graduation = 1 mL/s
- Radiopaque connecting ring
- Easy to check correct positioning
Other advantages include:
- Hybrid: titanium and POM(1) port for paediatrics and brachial placement
- Profile-shaped design
- Easy to connect
- Titanium-POM combination: low weight
- Titanium reservoir: compatibility with antineoplastic agents
- Radiopaque connecting ring
- MRI conditional & CT compatible