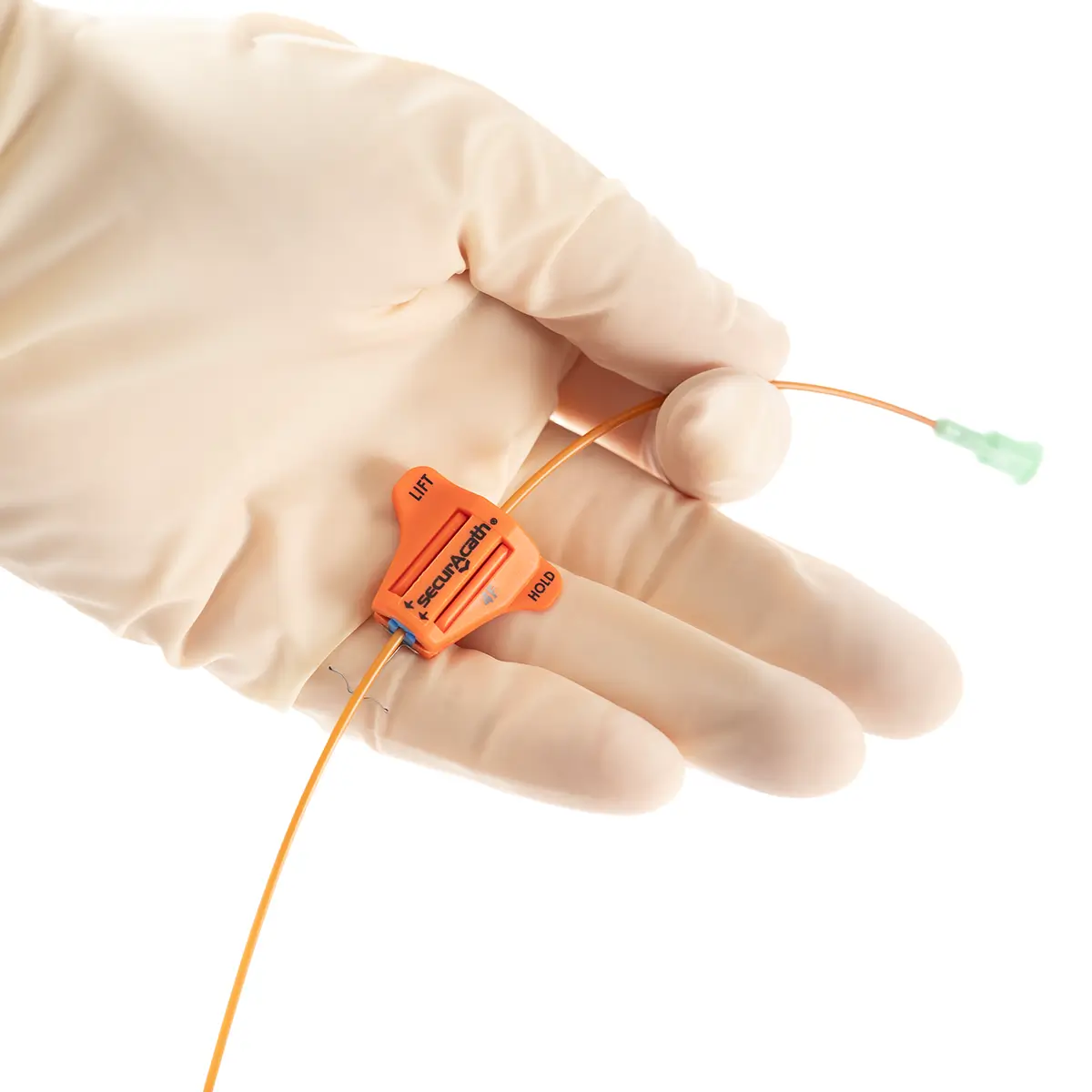
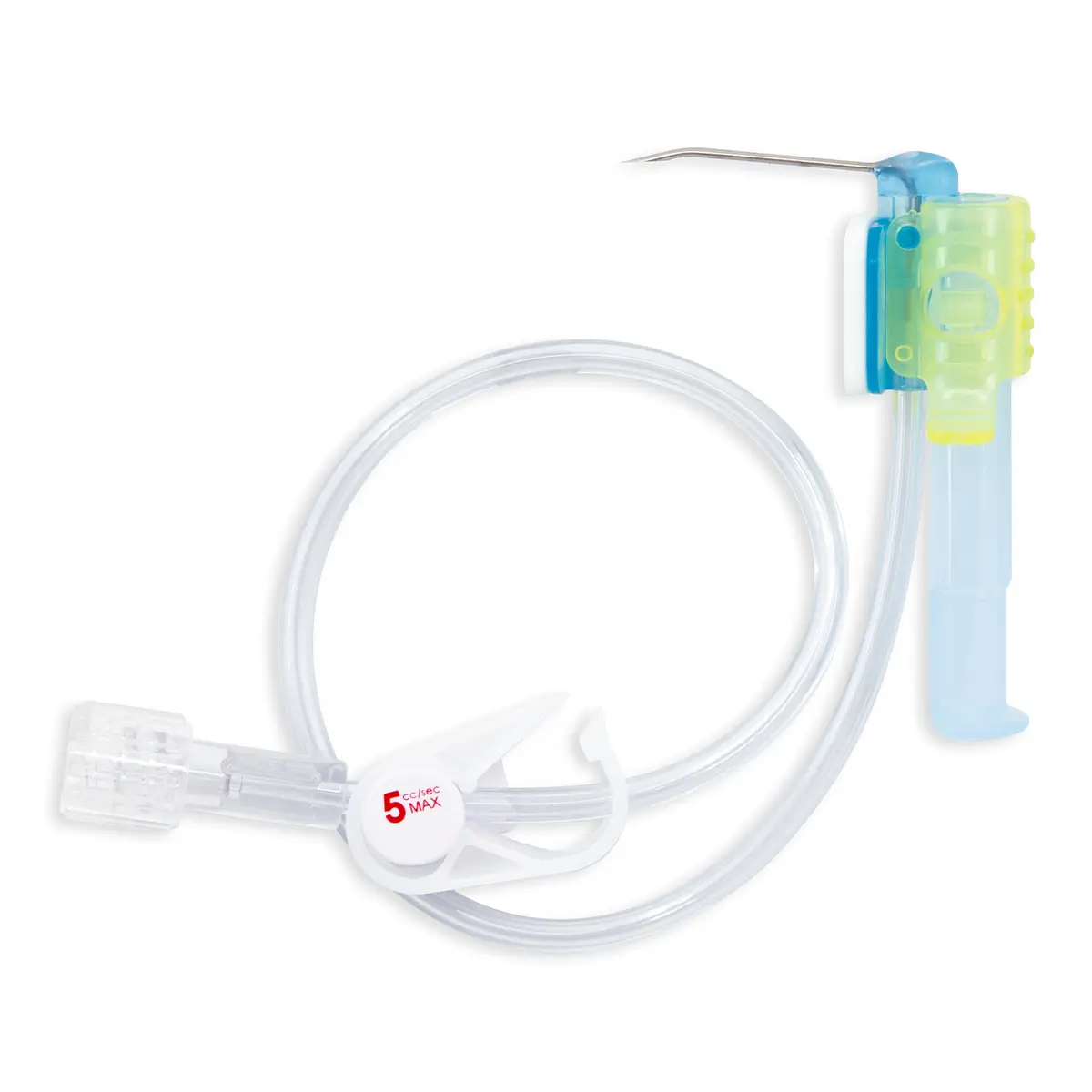
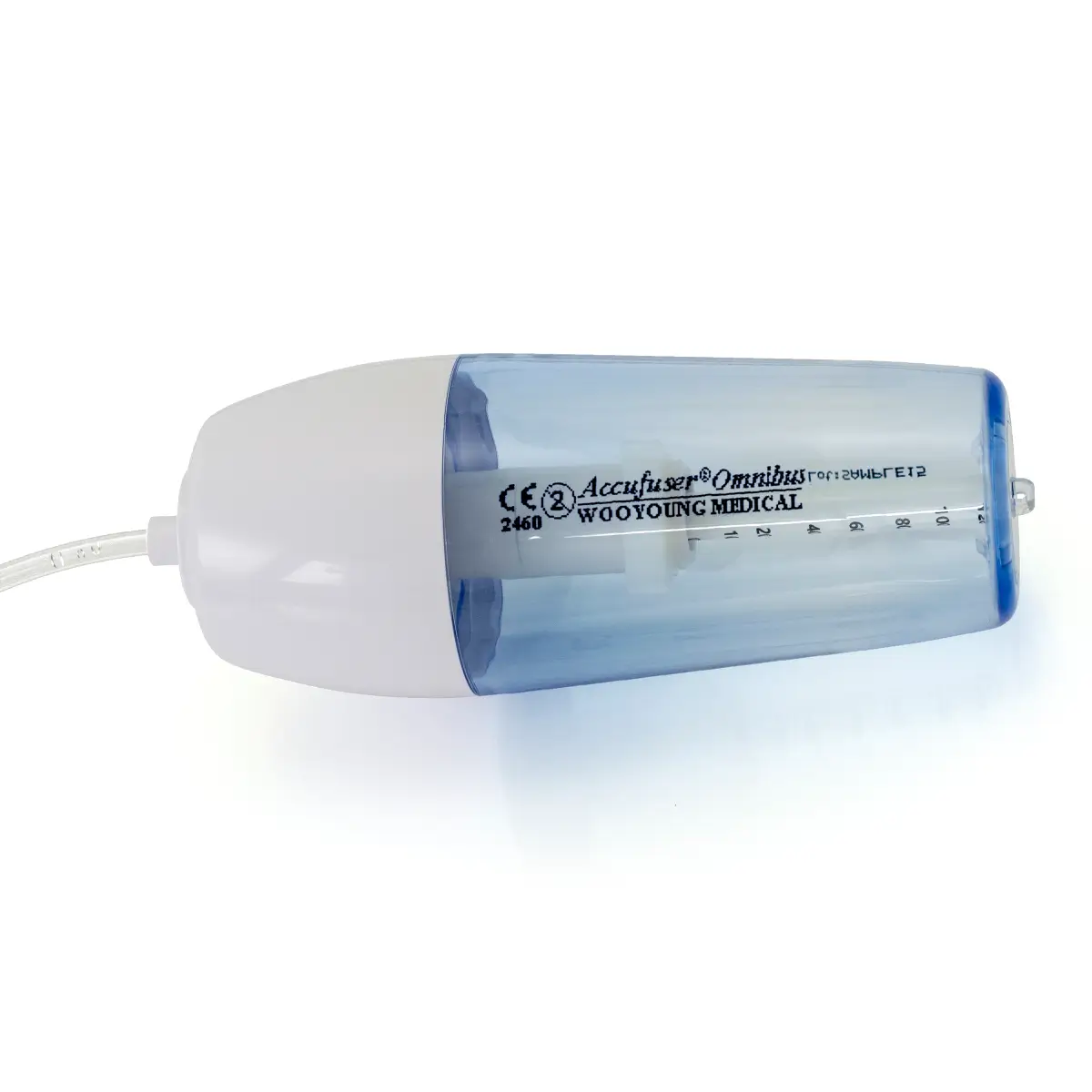
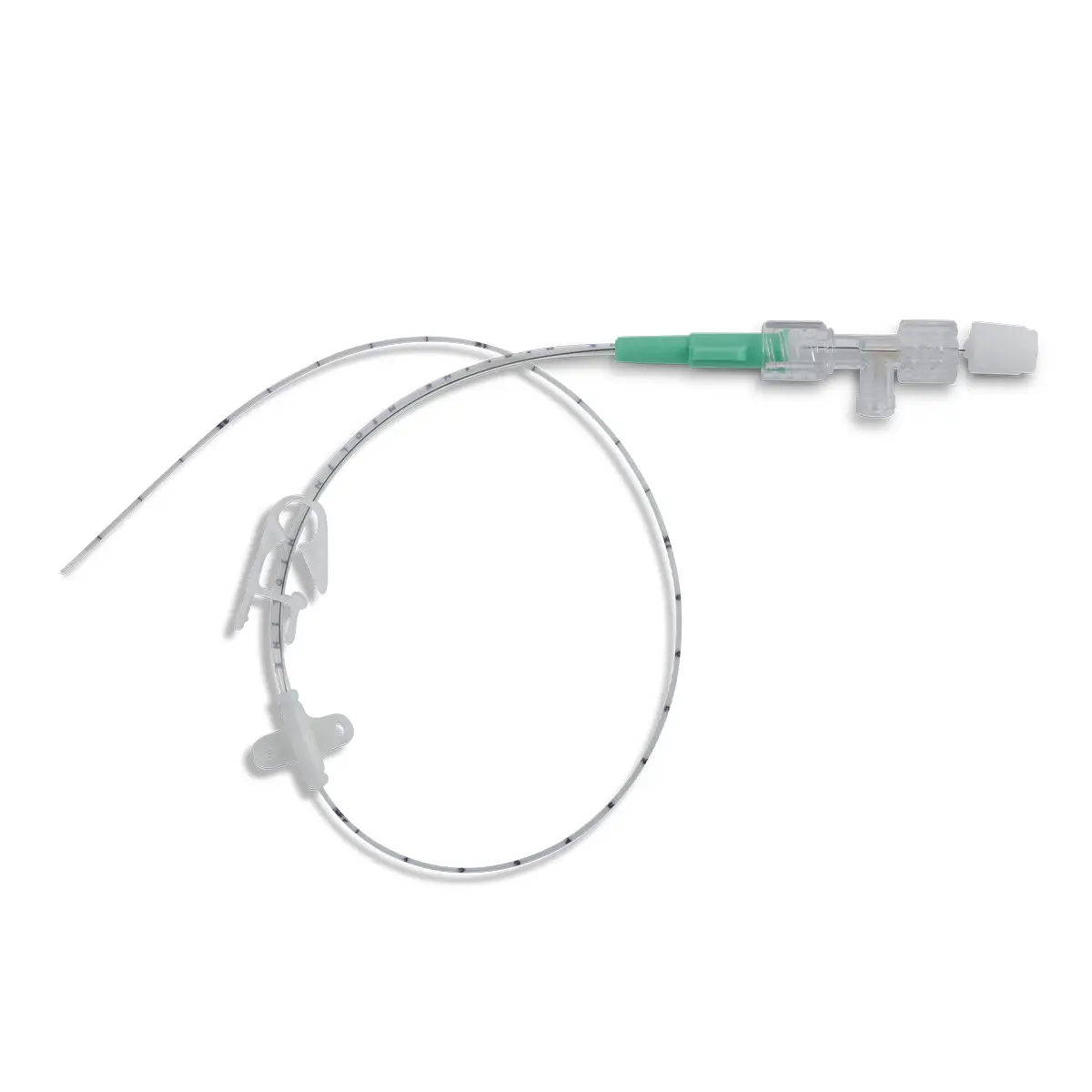
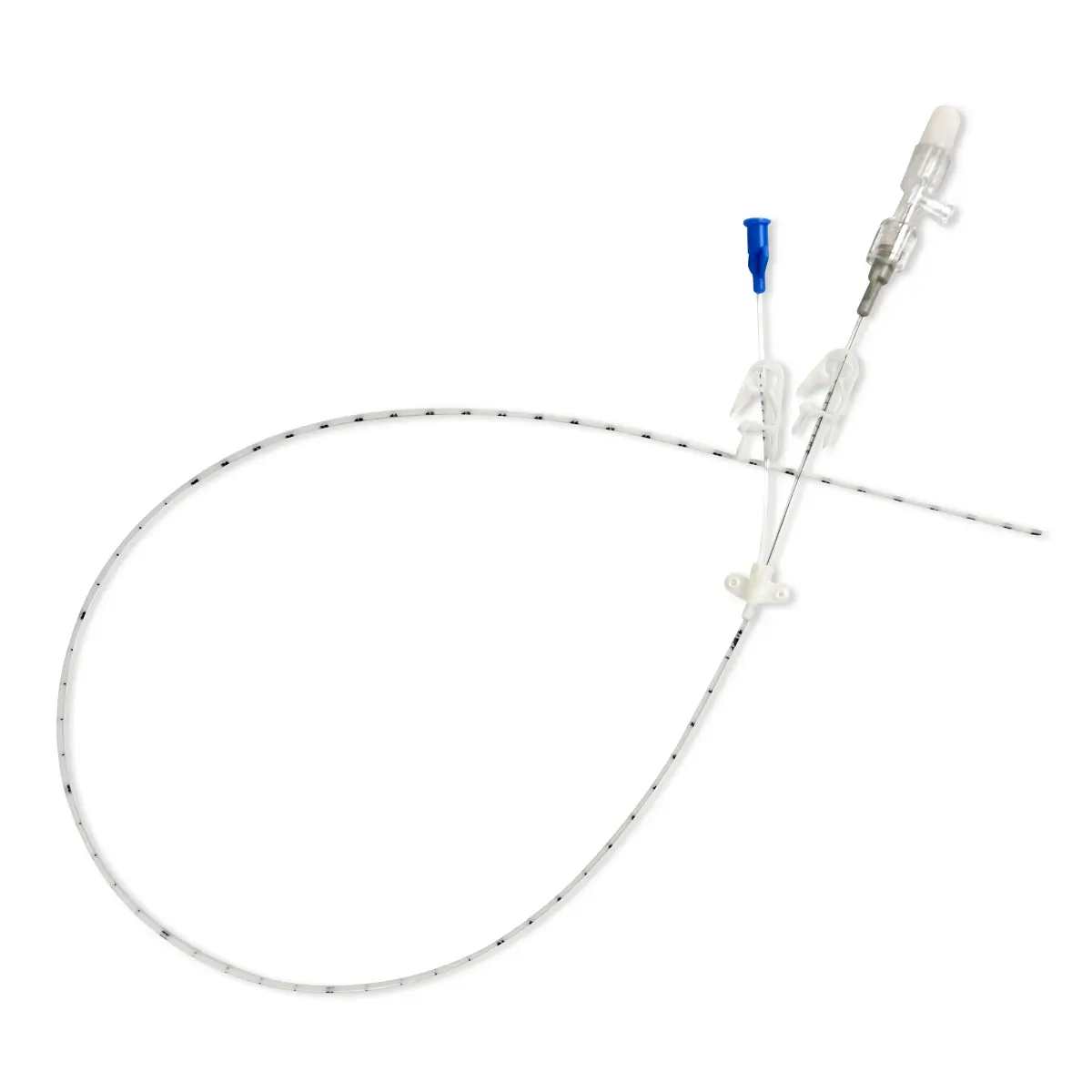
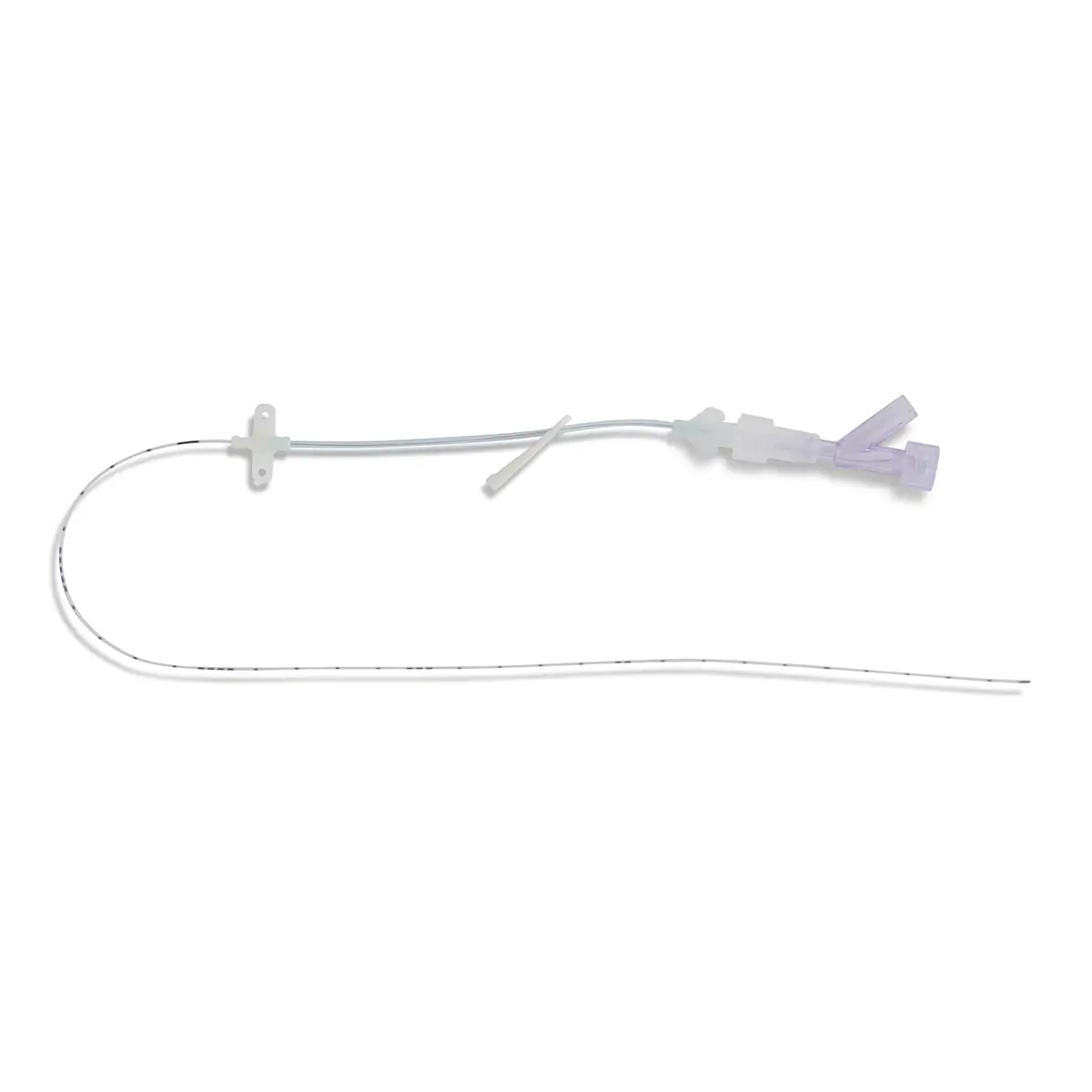
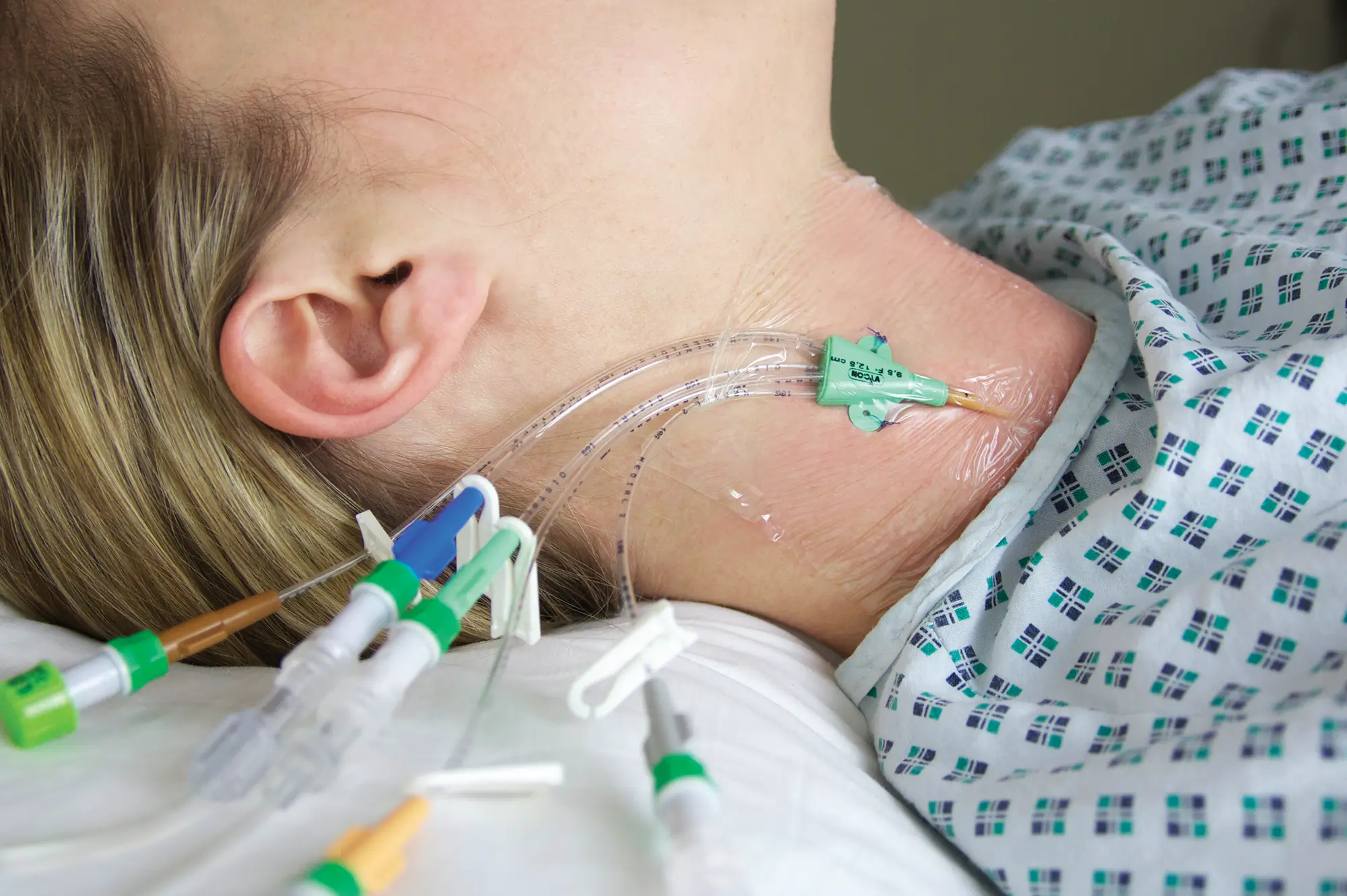
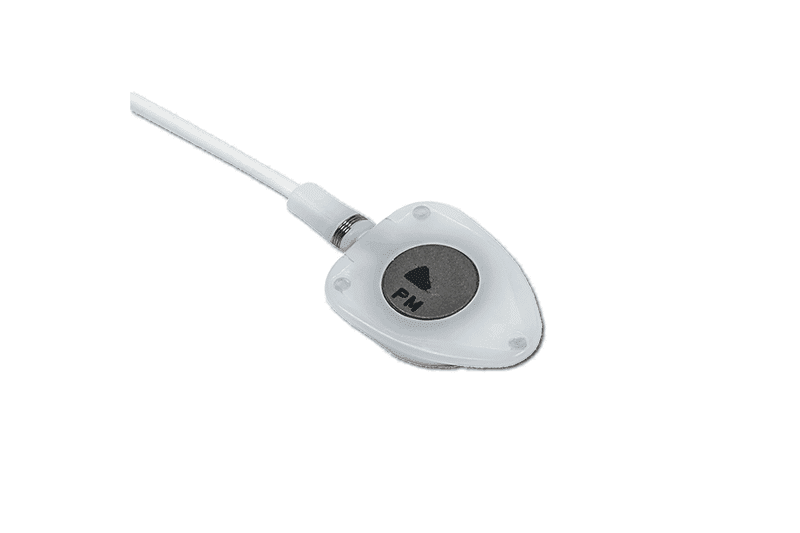
polysite™ is an easily administered implantable lightweight, hybrid port for adults requiring injectable medicines. It is positioned directly beneath the skin, usually in a discrete easily accessible place (chest level). Medicines then reach the blood circulation directly after simply puncturing the site through the skin.
Home / Vascular Access / polysite™ 4000 Series Standard Ports
Core Product Features
For larger adult patients, or high BMI patients
Focus on patient comfort during treatment
Lightweight design
Ergonomic shape for easy placement
Hybrid combination of titanium and POM
MRI conditional & CT Compatible
Access to Vygon clinical support
- Hybrid: titanium and POM(1) port for adults
- Profile-shaped design
- Easy to connect
- Titanium-POM combination: low weight
- Titanium reservoir: compatibility with antineoplastic agents
- Radiopaque connecting ring
- MRI conditional & CT compatible
UOI Units: Singles
UOI: 1
Contains Latex: No
Contains DEHP: No
Sterile Medical Device: Yes
Manufacturer: Vygon SA
Vygon is committed to education and support for our customers. We provide in-service training sessions for all staff and all shift patterns, to help ensure your clinicians achieve best practice when delivering care to patients. In addition, patient advice leaflets and children’s bravery certificates are available on request, to help assist the clinicians in supporting and informing their patients.