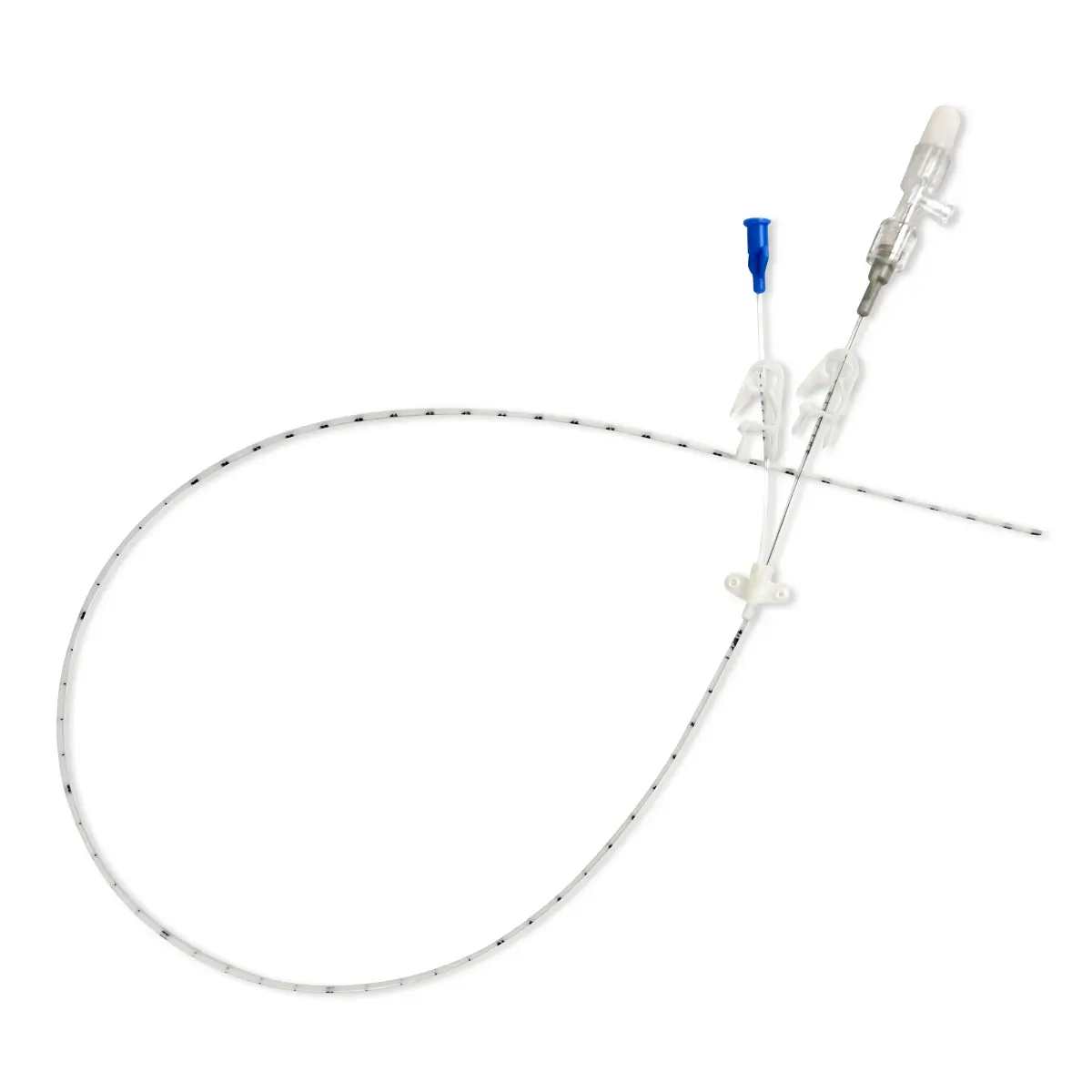
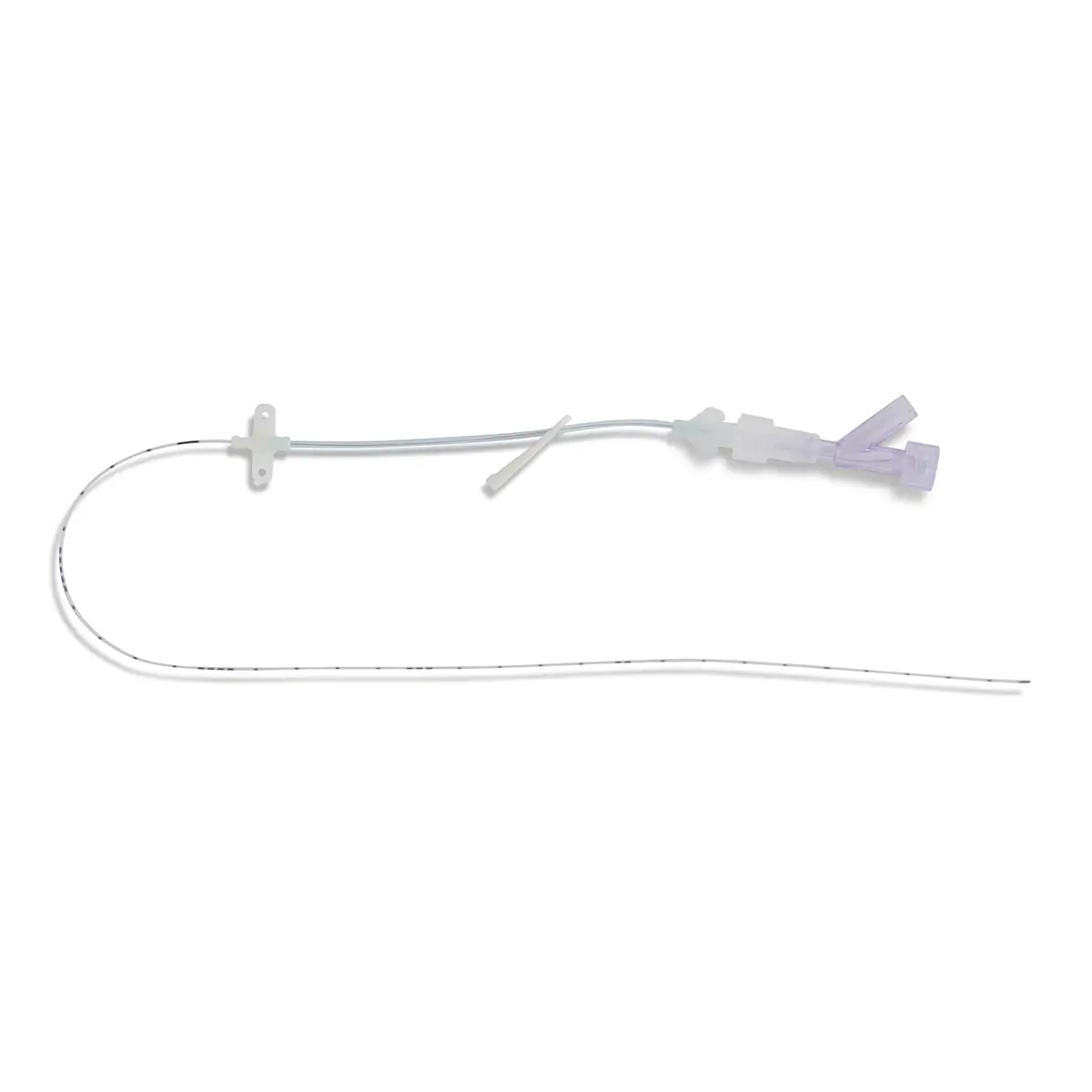
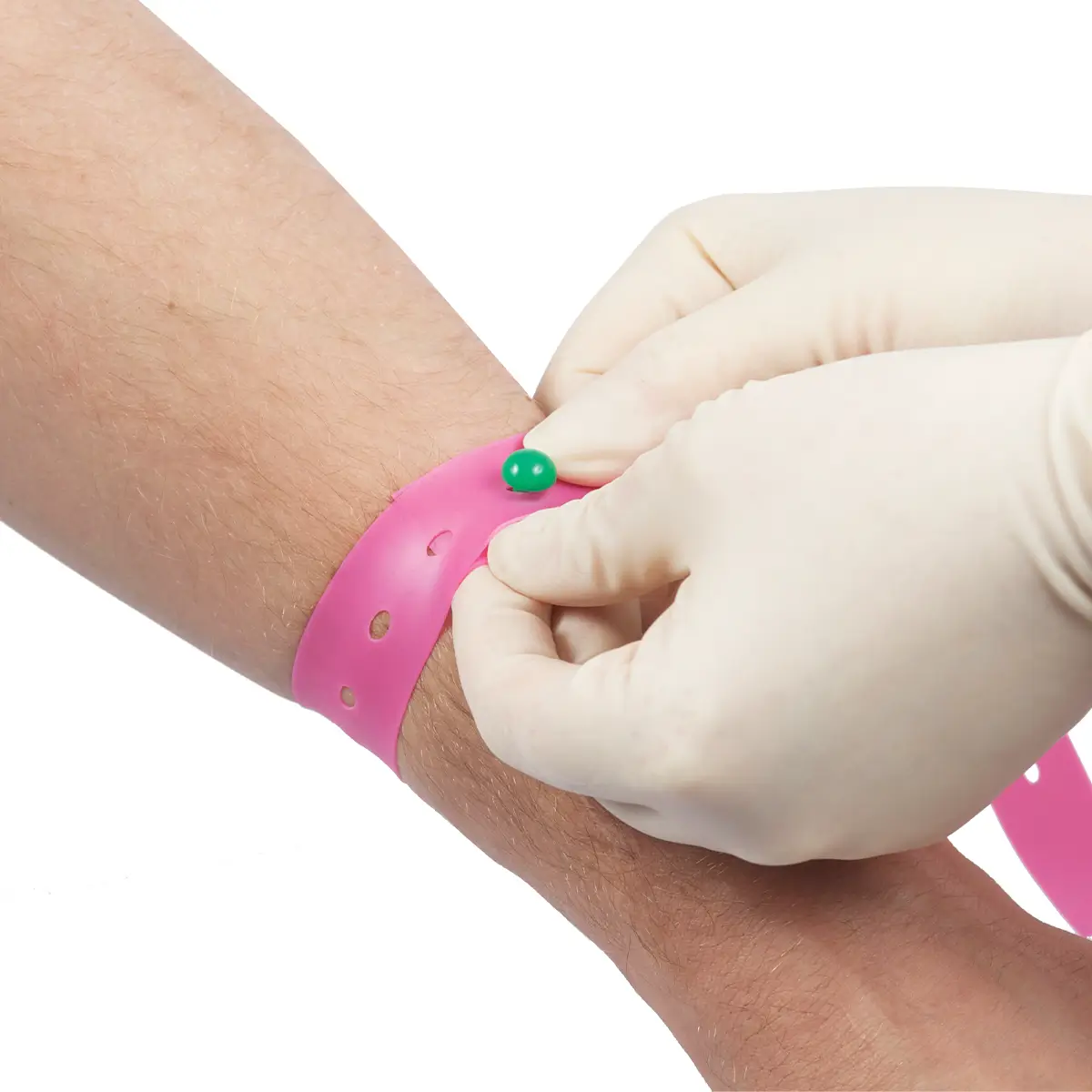
Vene-K is a silicone single-use, quick-release tourniquet.
Vene-K is intended to avoid cross-contamination due to the fact that a high proportion of reusable tourniquets are contaminated with blood and bacterial pathogens.

Tourniquet