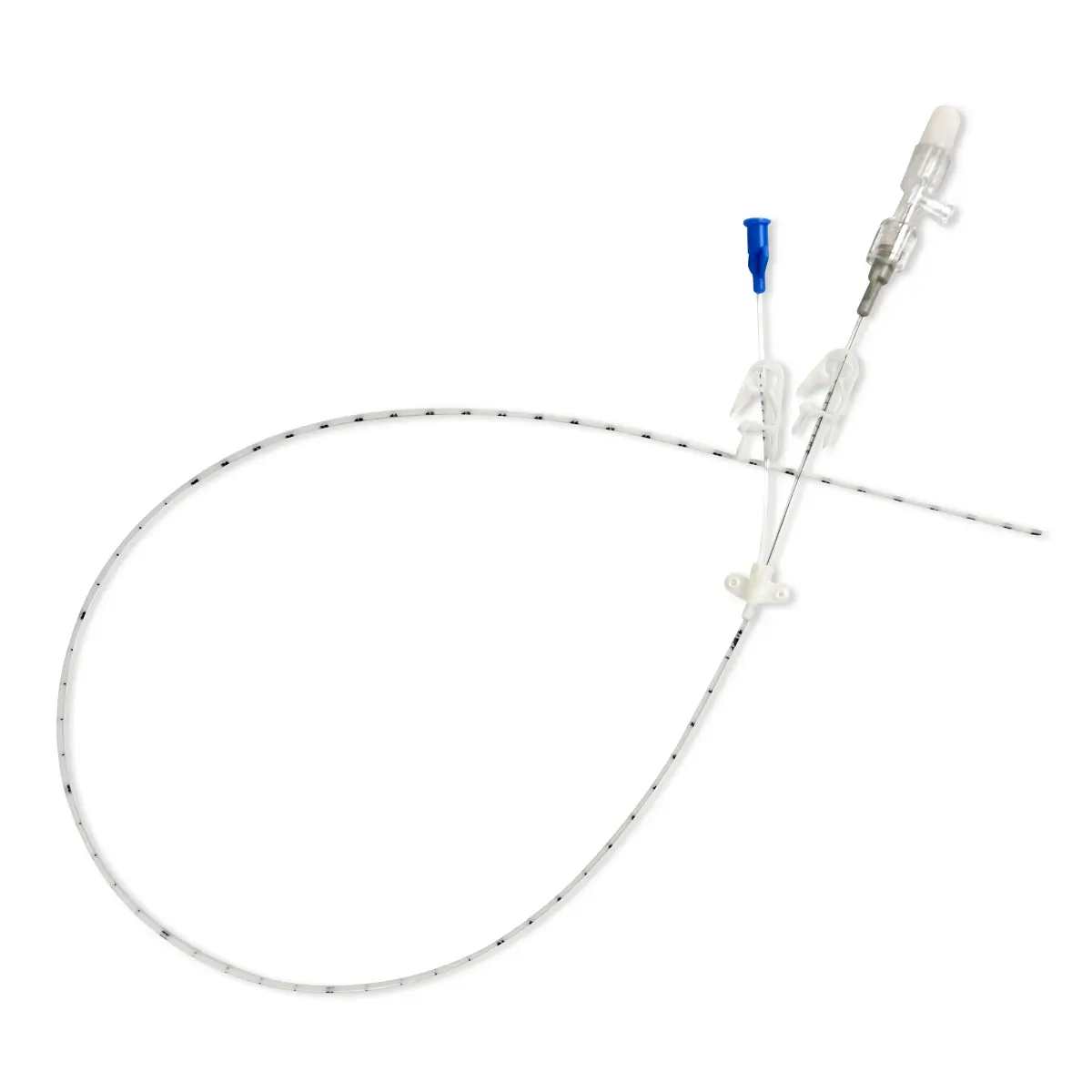
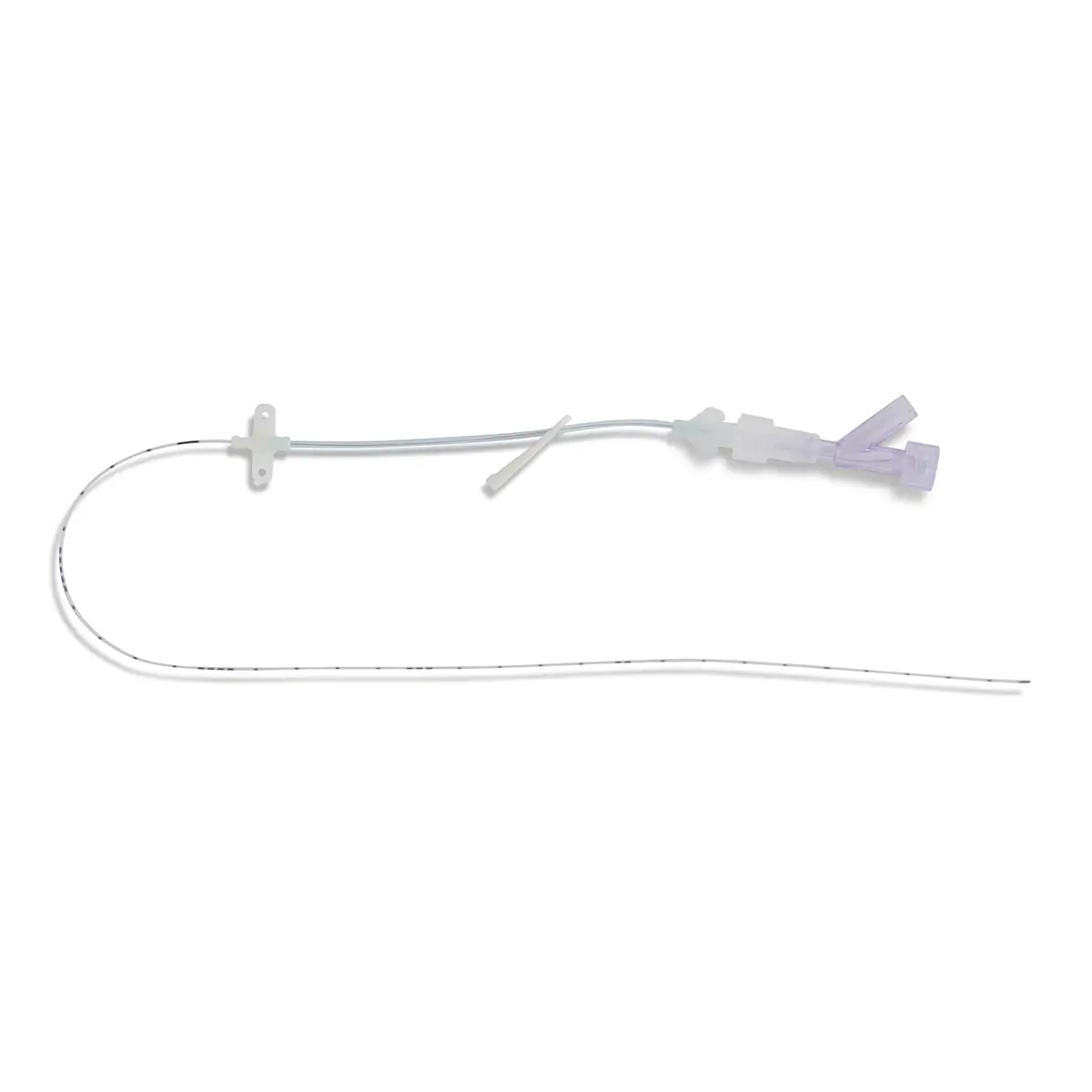
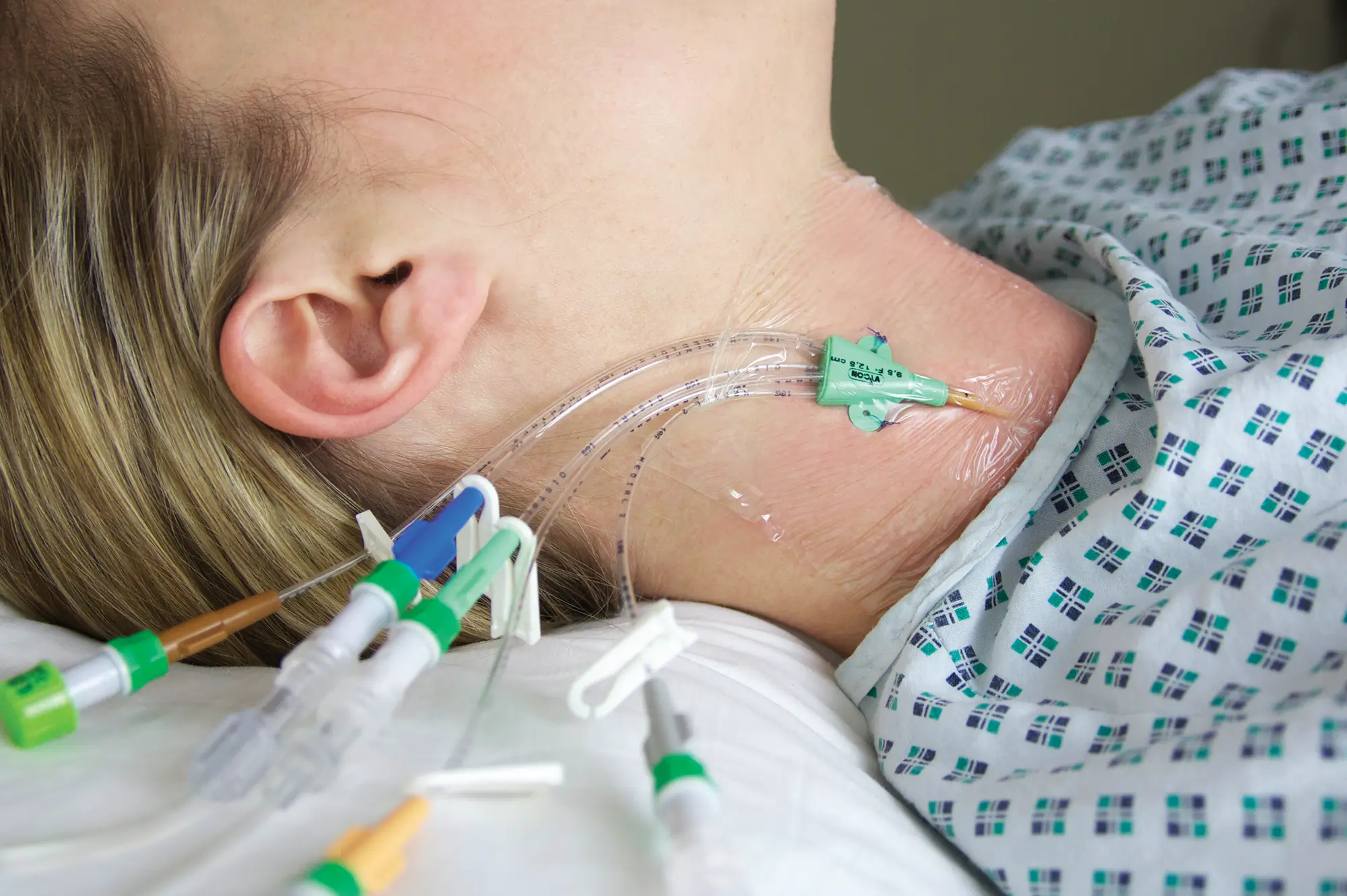
Bionector is a market-leading needlefree connector, designed to meet and exceed global opinion leader’s recommendations for reducing catheter-related bloodstream infections (CRBSI’s). Proven to provide an effective barrier against microbial ingress, and help standardise practice by combining a fixed, straight fluid pathway with innovative neutral displacement technology.
CDC 2011 guidelines suggest that needle-free devices address occlusion problems by incorporating neutral fluid displacement. (3)
Bionector Needless Connector – Benefits to Patients and clinicians
- Fast and safe access to the infusion line
- Reduce the risk of needle stick injuries and exposure to blood reflux
- Reduce the risk of catheter-related bloodstream infections
- Reduction in risk of device occlusion, air embolism and blood loss
- Help to prevent inadvertent disconnections and leaks in the infusion system
Neutral Fluid Displacement & No Post-Flushing Clamping Sequence:
Bionector leads the way with neutral fluid displacement. It is the only neutral displacement needle free connector to combine a split-septum with a fixed, straight fluid pathway. The neutral displacement means that a specific post-flushing camping sequence is not required, which in turn helps prevent blood reflux and reduce catheter occlusions.
Easy to Clean & Clear
The smooth split-septum fits tightly into the device housing, free from any gaps. The straight, fixed, fluid pathway has been proven ‘easy to clear’ and is designed to provide the most direct and least tortuous route with no moving parts (such as mechanical valves). These design features reduce surface area for biofilm formation.
Low Deadspace
The Bionector straight fluid pathway is proven ‘flushable’ for macro and microscopic particles such as blood. With minimal deadspace of just 0.03ml, allowing for a low flushing volume (5ml) to clear the device.
MRI Conditional and CT-rated
- Tested and proven not to represent any risk to ether patients or practitioners during an MRI of up to three Teslas.
- CT-rated for use with power injectors
- Maximum pressure resistance of 350psi and a maximum flow rate of 10ml/s
Clinically Proven
Bionector is backed up by a robust library of clinical studies. For more information on relevant clinical performance studies please contact a member of the Vygon team.
Total Solutions: Training, Support and Education
Bionector is a core part of our the Vygon UK Total Solutions package. With a dedicated training and education program, combined with clinical tools, services and solutions to support your healthcare professionals and patients. Find out more about Total Solutions.
Suitability:
Bionector Needless Connector can be used in a variety of clinical settings where intravenous access is required. Suitable for blood sampling, intermittent injections or continuous infusions of fluids or drugs. Can be used in both hospital and for longer-term outpatient treatment.
Can be used with a range of CVCs, PICC’s, Midlines from Vygon UK.
Product Features
References
- Bionector Fluid Displacement Test, report 200700807, Rev 01, Nelson Laboratories USA, 19th April 2007.
- Food and Drug Administration Agency (FDA), ‘Guidance for Industry and FDA staff’: Pre-market notification submissions, Microbial Ingress Testing, section 8, page 9, July 11th 2008
- Centre for Disease Control, ‘Guidelines for the Prevention of Intravascular Catheter-Related Infections’, Needleless Intravascular Catheter Systems, page 19, No.6. 2011.
- https://www.ins1.org/publications/infusion-therapy-standards-of-practice/