Bionector is an established market-leading needle-free device which meets the full range of global opinion leaders’ recommendations for reducing CRBSIs. It has been proven to provide an effective barrier against microbial ingress and help standardise practice by combining a fixed, straight fluid pathway with innovative neutral displacement technology. And it is the only neutral displacement needle-free device in the UK to combine a split-septum with a fixed straight, fluid pathway.
Benefits:
Bionector leads the way with a neutral fluid displacement.This means a specific post-flushing clamping sequence is not required, which in turn helps prevent blood reflux and reduce catheter occlusions. It is also
backed up by a robust library of clinical studies, Bionector is proven to be easy to clean and clear. Its smooth split-septum fits tightly into the device housing ensuring it is free from any gaps.The straight, fixed fluid pathway has been proven ‘easy to clear’, designed to provide the most direct and least tortuous route with no moving parts (such as mechanical valves), which reduces the surface area available for biofilm formation.
Aswell as being proven not to represent any risk to either patients or practitioners during an MRI of up to threeTeslas. CT-rated for use with power injectors Bionector has a maximum pressure resistance of 350psi and a maximum flow rate of 10ml/s. Plus, Bionector’s straight fluid pathway is proven ‘flushable’ for macro and microscopic particles such as blood.This is due to a minimal deadspace of just 0.018ml allowing for a low flushing volume (5ml) to clear the device. Clean in accordance with your hospital/department protocol; Bionector can be cleaned with most disinfecting agents (eg 70% alcohol). Avoid antiseptics containing both alcohol and hydrogen peroxide.
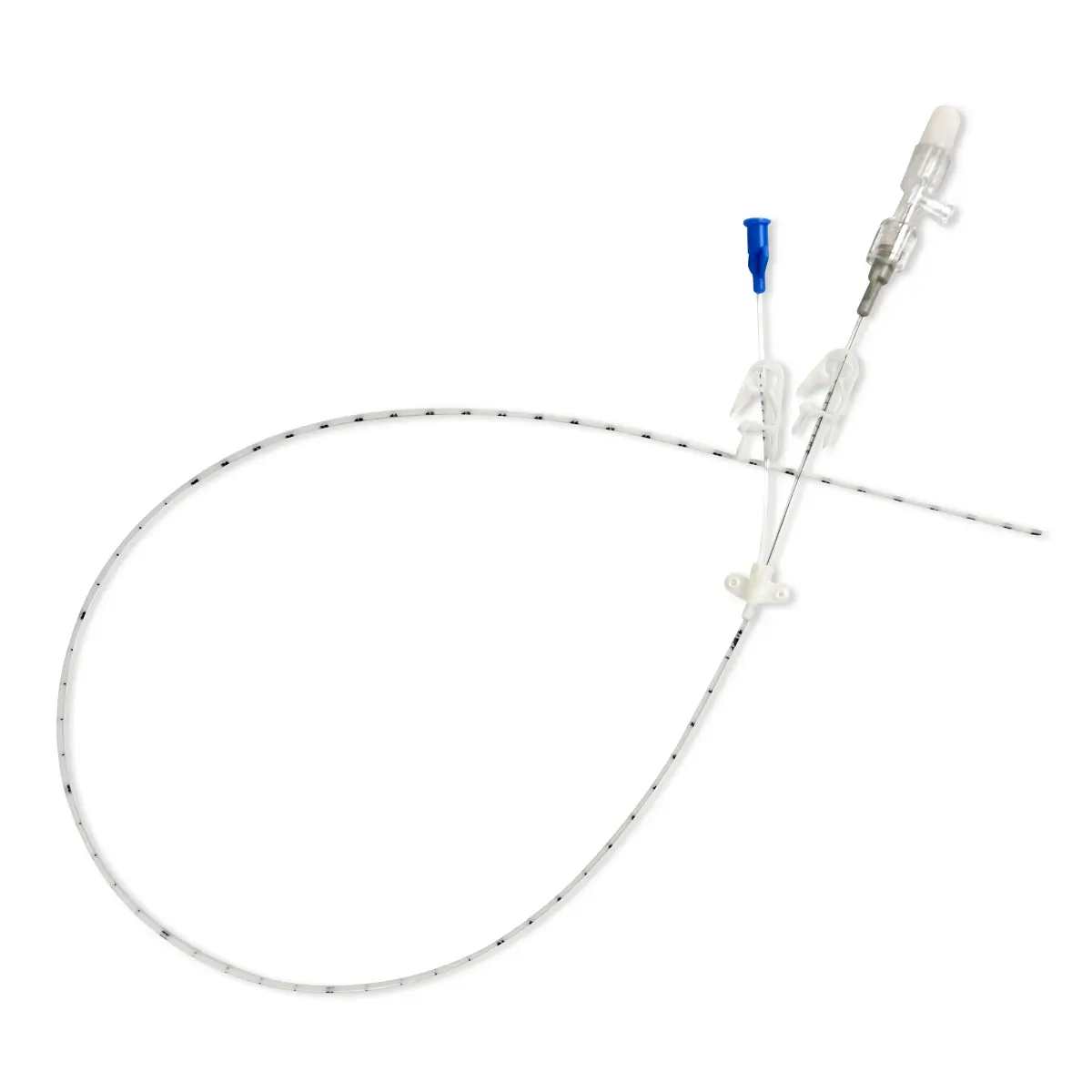
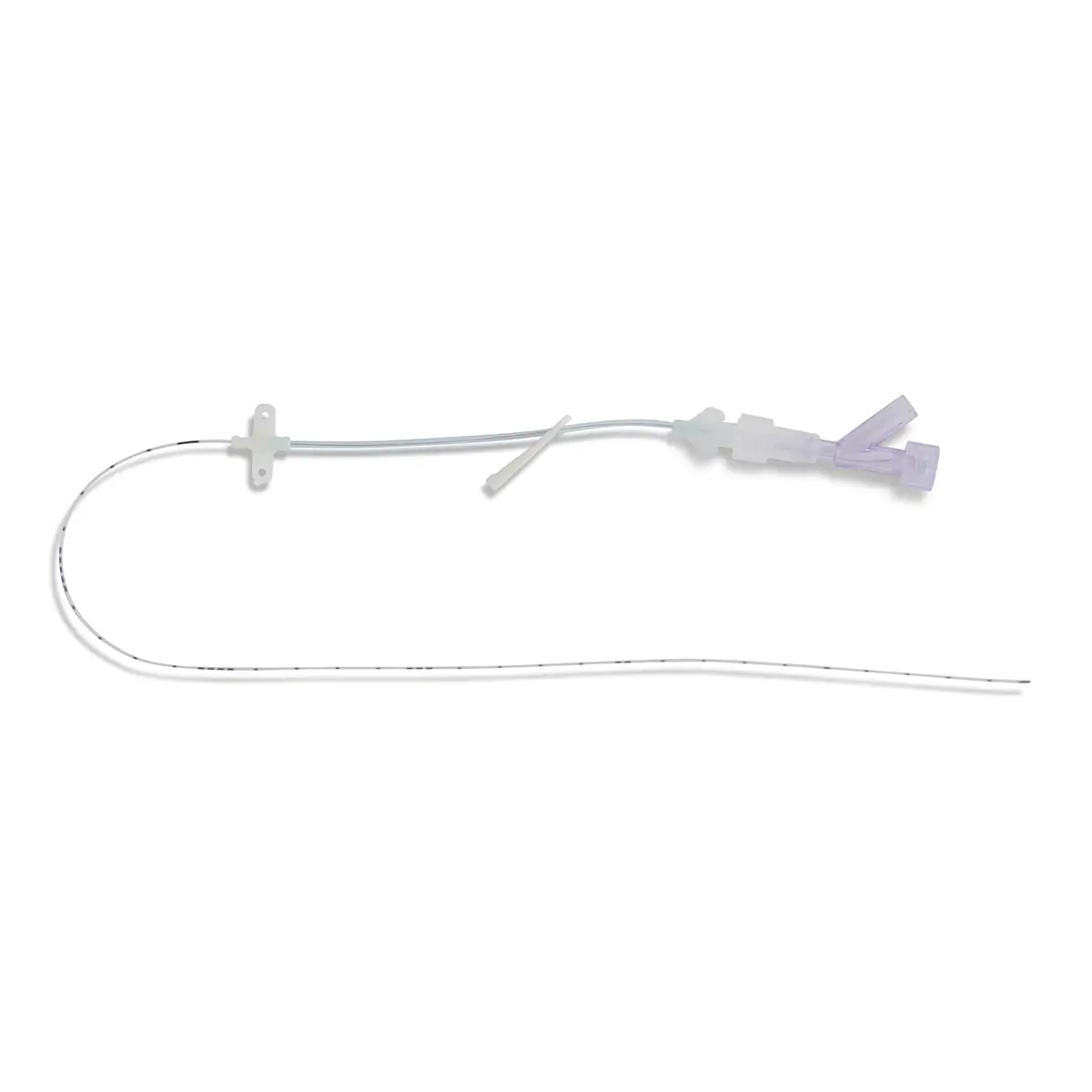
Arterial Bionector Closed System Needlefree Device in soft pack
Arterial Bionector (code 896.11 and 896.31) is a needleless connector featuring on its distal end (green) a male Luer-lock connection. This male luer-lock end is connected on the side port of a 3-way stopcock fitted on an arterial monitoring line, or on the extension tube of an arterial line.
The proximal end (red) consists of a female Luer-lock port which is kept in a closed position by a pre-split membrane. When the male Luer or male Luer-lock port of a syringe is connected to the Bionector, the pre-split membrane moves along a metallic tube thanks to a spring. The device is then in an open position allowing blood sampling and/or the flush of the Bionector. On removal of the syringe, the spring pushes the membrane backwards closing the device.
Clean in accordance with your hospital/department protocol. Bionector can be cleaned with most disinfecting agents (eg: 70% alcohol). Avoid antiseptics containing both alcohol and hydrogen peroxide.
Bionector is resistant to lipid emulsions (lipid-resistant), most cytotoxic drugs and antiseptics. This simple to use device protects both health care workers (prevents needle-stick injury, no blood reflux) and patients (closed system: reduces risk of air embolism and blood loss).
Bionector features a male Luer-lock distal fitting which means that Bionector can be connected to all devices featuring a female Luer-lock hub (IV catheter, extension tubes, infusion devices for implantable ports, ramps, stopcocks…). The proximal end of Bionector is a female Luer-lock fitting and will accept any device featuring a male Luer or male Luer-lock fitting (syringe, infusion set).
Bionector is a needleless connector with reverse split-septum technology and neutral displacement of volume.
Bionector is approved for use with power injectors (CT-scan).
Maximum pressure resistance : 350 psi (24 bars).
To inject contrast medium products (Visiopaque 320 mg iodine/ml), the flow rate has been measured as such :
35 psi (2.4 bars) : > 5 ml/s
145 psi (10 bars) : > 10 ml/s