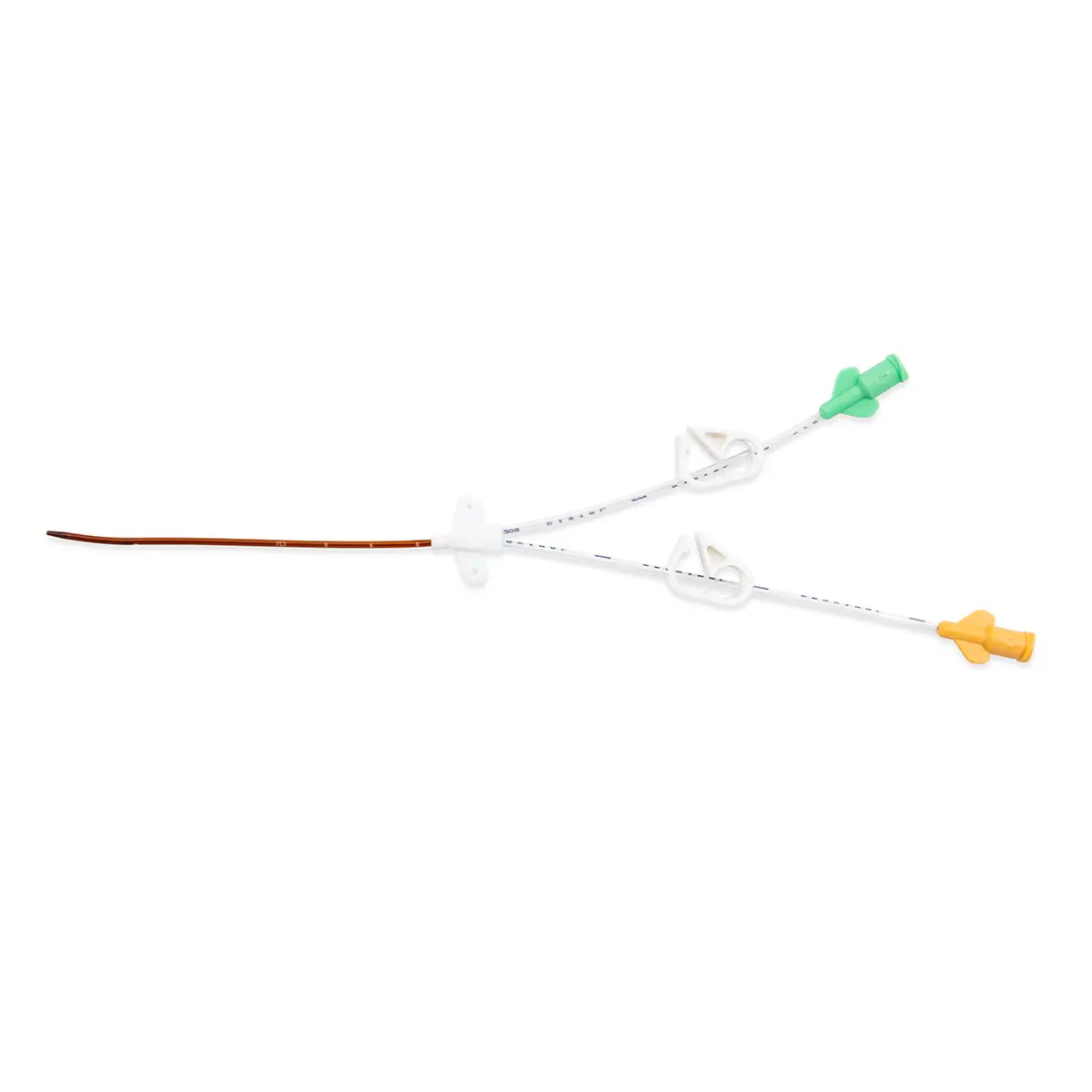
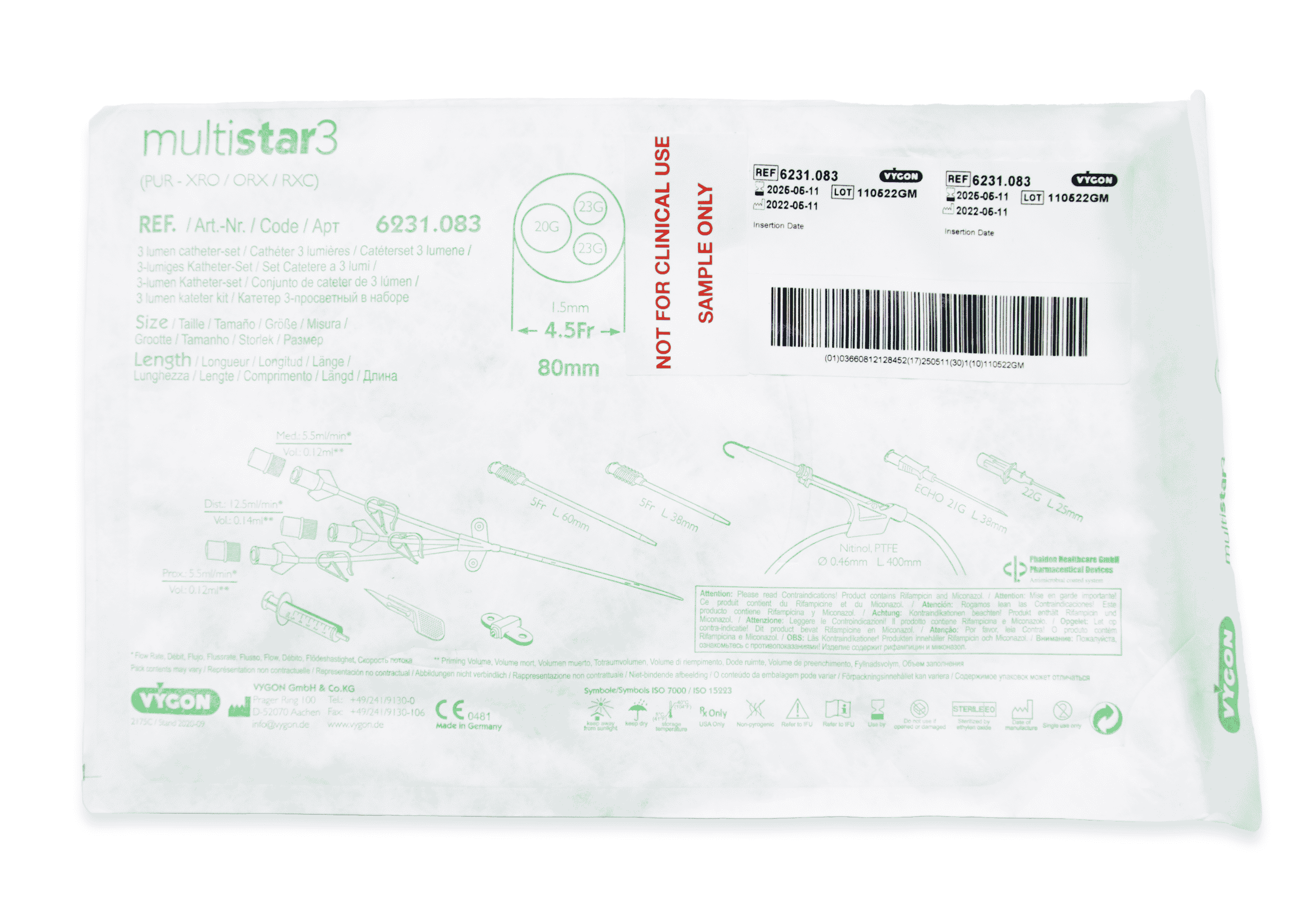
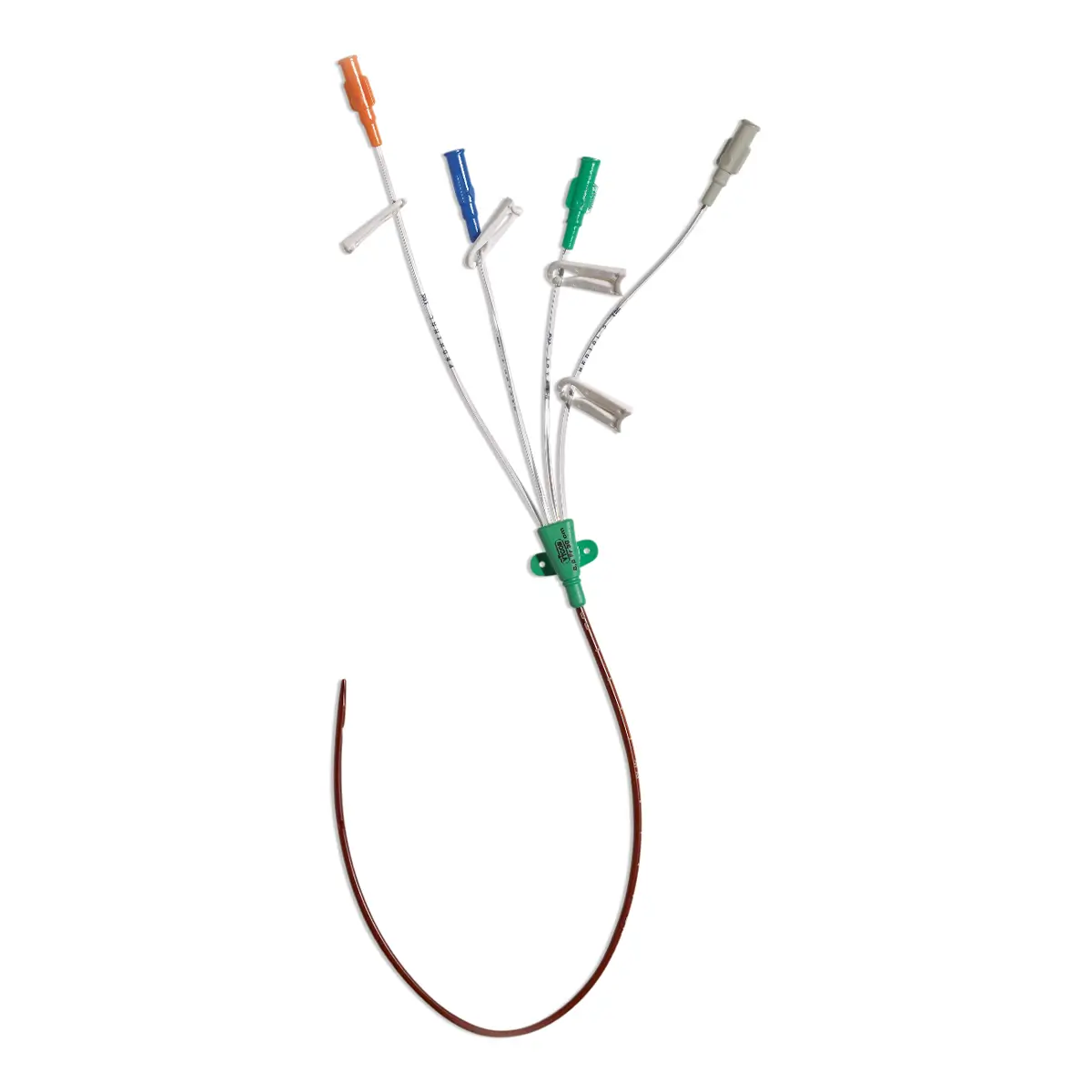
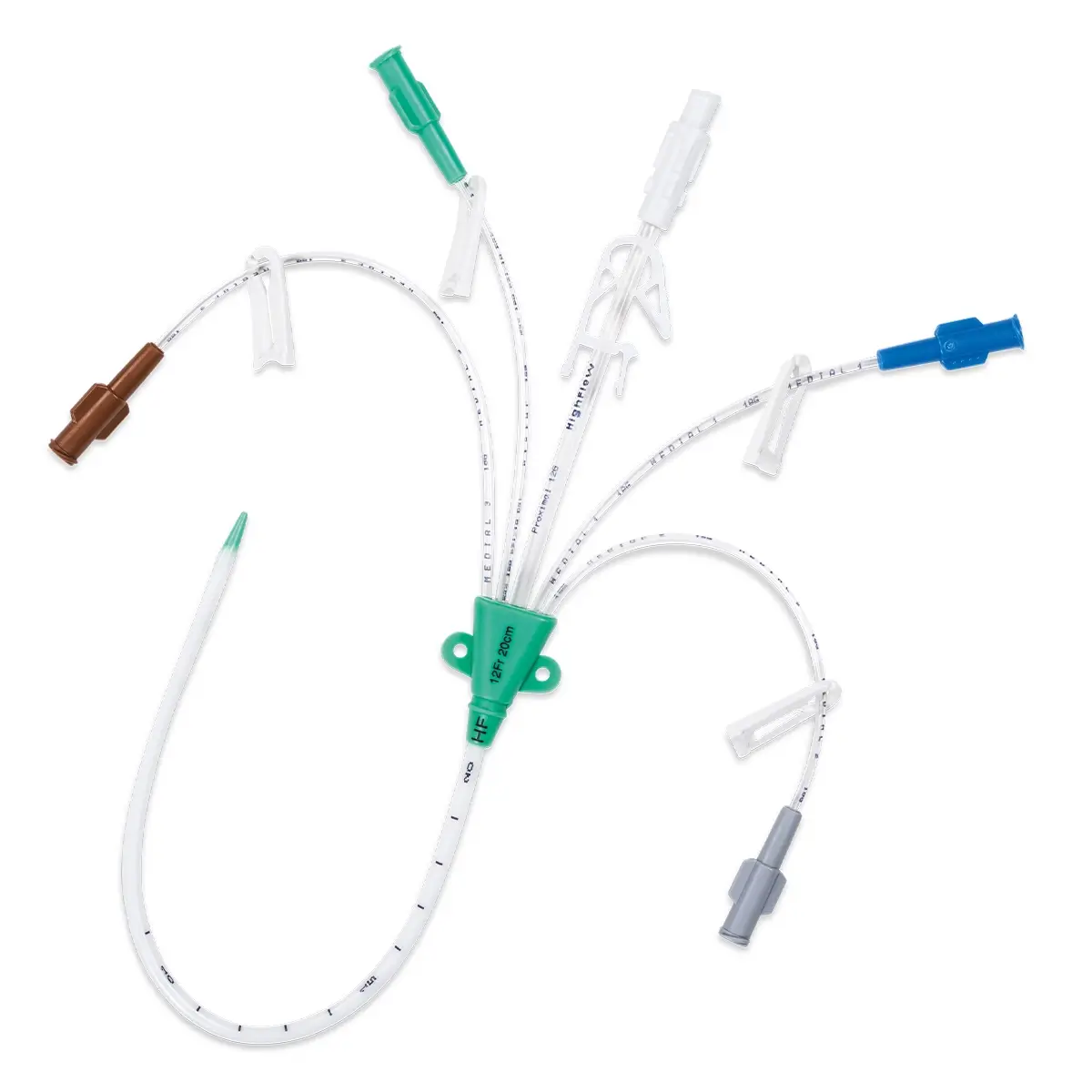
Multistar Paediatric Central Venous Catheter
Specially designed for even the most fragile of patients, the Multistar Paediatric CVC is puts treatment of children at the forefront.
Leading the Way in Paediatric Design for Central Venous Catheters
Select the right catheter for every patient with Vygon Multistar. Choose the best size CVC for the patients’ requirements, depending on their age, weight and therapy needs with a range of dilator length, catheter lengths and lumen numbers.
The Vygon Multistar CVC has multiple features specially designed with children in mind:
- Antimicrobial incorporated Multistar clinically proven to reduce CRBSI
- Two vessel dilator lengths for added safety on catheter insertion
- Miniature size better suited to paediatric anatomy
- Flexible tip protects the vessel walls
- X-ray opaque catheter for confident tip placement
- “J” Nitinol guidewire coated with Teflon for ultra-low resistance insertion
- A choice of a short IV cannula or a high-performance echogenic introducer needle for catheterisation
- Roberts clamps for secure one-handed line clamping
Enhanced Patient Safety with Antimicrobial Technology
The Multistar Paediatric CVC leads the way with antimicrobial technology for enhanced patient safety, by reducing the risk of catheter microbial colonisation and catheter related blood stream infections (CRIBSI’s).
Multistar uses the innovative combination of two active ingredients: Rifampicin and Miconazole, which are molecularly integrated into the polyurethane catheter material by using a patented multistep diffusion-controlled incorporation process. This combination leads to protection against a broad spectrum of microorganisms such as Staphylococci, Enterobacterial and candida. (2)
“Rifampicin-Miconazole supersaturated CVCs have demonstrated the potential to prevent catheter-associated colonization, local infection and bloodstream infection even in long-term application.”(3)
Multistar is the innovative combination of two active ingredients: Rifampicin and Miconazole, chosen for their synergic properties:
- Efficacy on a large spectrum of microorganisms (3)
- Low risk of bacterial resistance development (3)
Clinically Proven
Multistar Paediatric CVC is backed up by a robust library of clinical studies. For more information on relevant clinical performance studies please contact a member of the Vygon team.
Training, Support and Education
Multistar Paediatric CVC is a core part of the critical care range of central venous access devices from Vygon UK. With access to dedicated training and education, combined with clinical tools, services and solutions to support healthcare professionals and patients.
References
- Scott-Warren, VL, Paediatric Vascular Access, BJA Education 15: 199-206, 2015
- Schierholz J, Nagelschmidt K, Nagelschmidt M, Lefering R, Yücel N, Beuth J, Antimicrobial central venous catheters in oncology: efficacy of a rifampicin-miconazole-releasing catheter, Anticancer Research 30: 1353-1358, 2010.
- Schierholz J, Summary of the issues associated with catheter related blood stream infections with special consideration given to catheters incorporating Rifampicin and Miconazole, 2011.
- Trieschmann, U. Udink ten F Cate. Sreeram, N. Central venous catheters in children and neonates – what is important?, Images in Paediatric Cardiology 9: 1-8, 2007.
- Rump A.F.E, Güttler K, König D.P, Yücel N, Korenkov M, Schierholz J, Pharmacokinetics of antimicrobial agents rifampicin and miconazole released from a loaded central venous, Journal of Hospital Infection 53; 129-135, 2003.