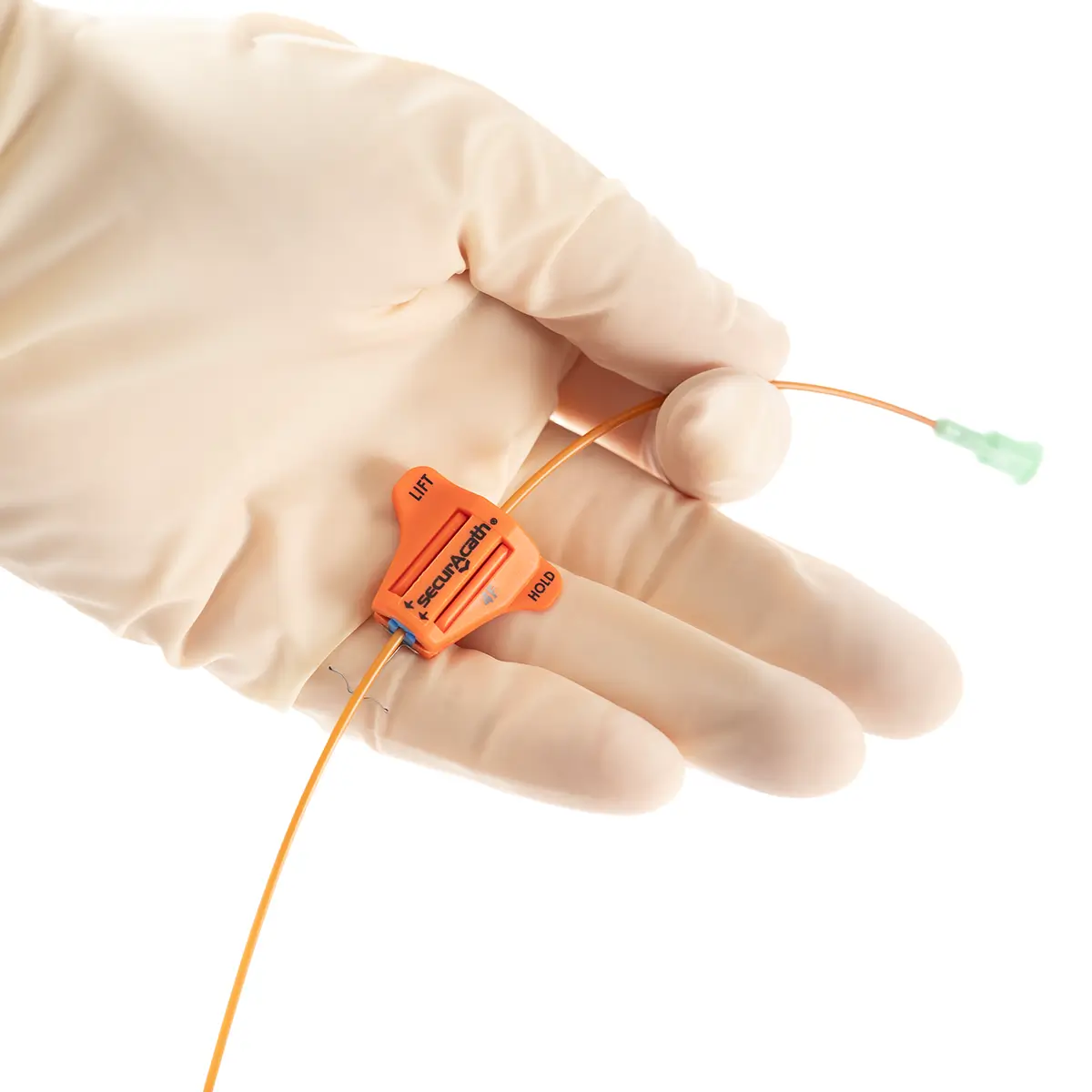
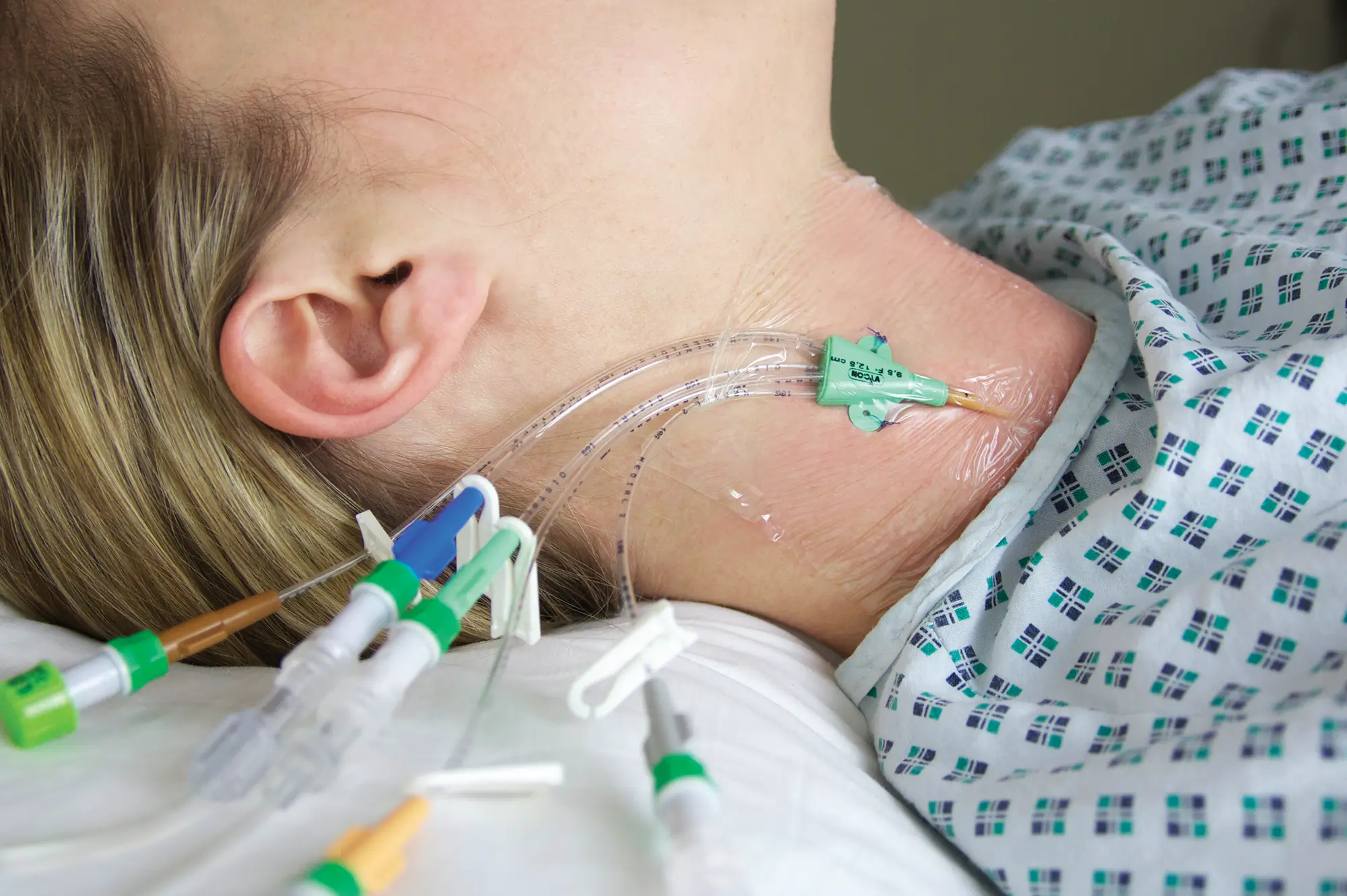
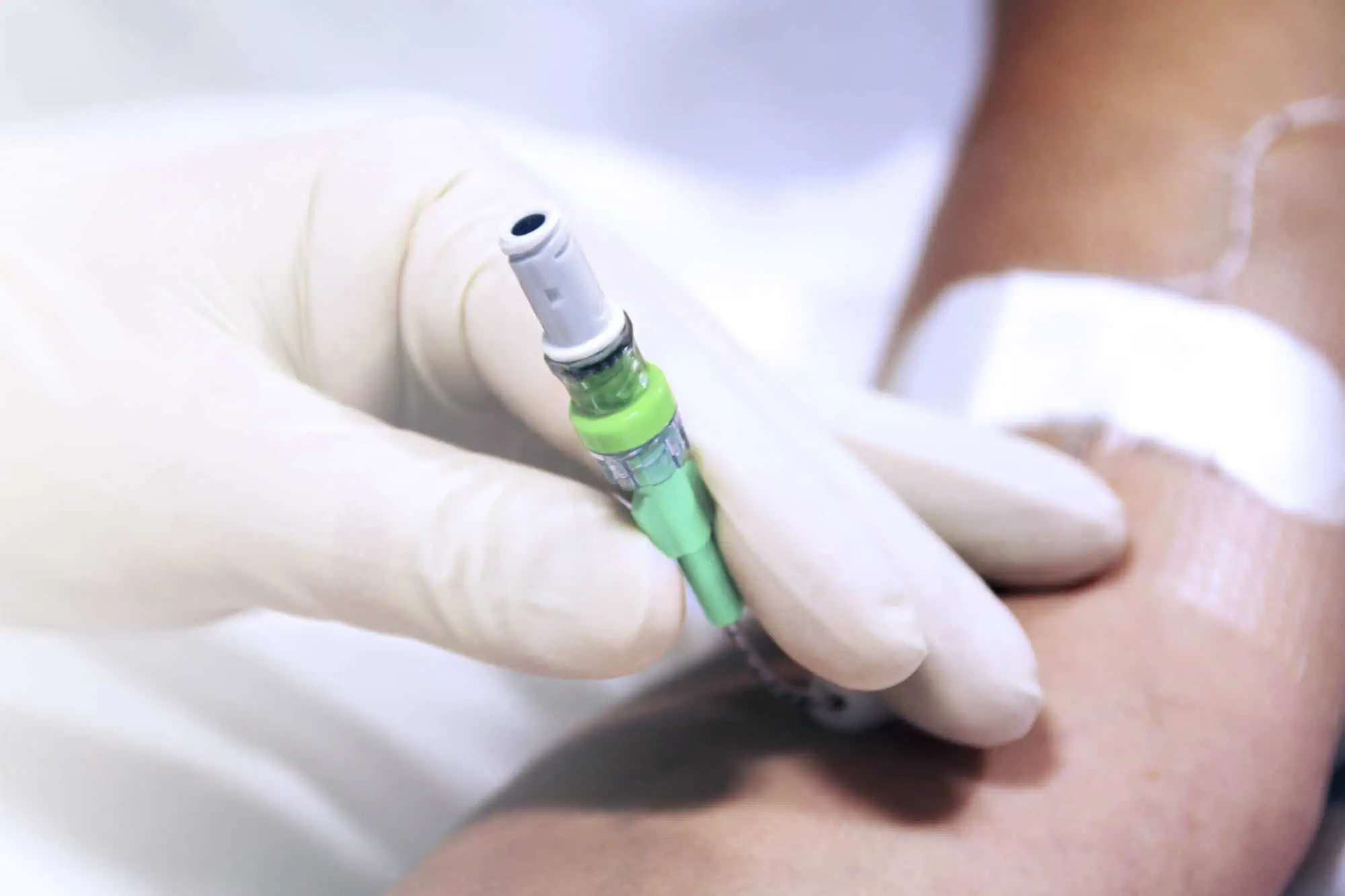
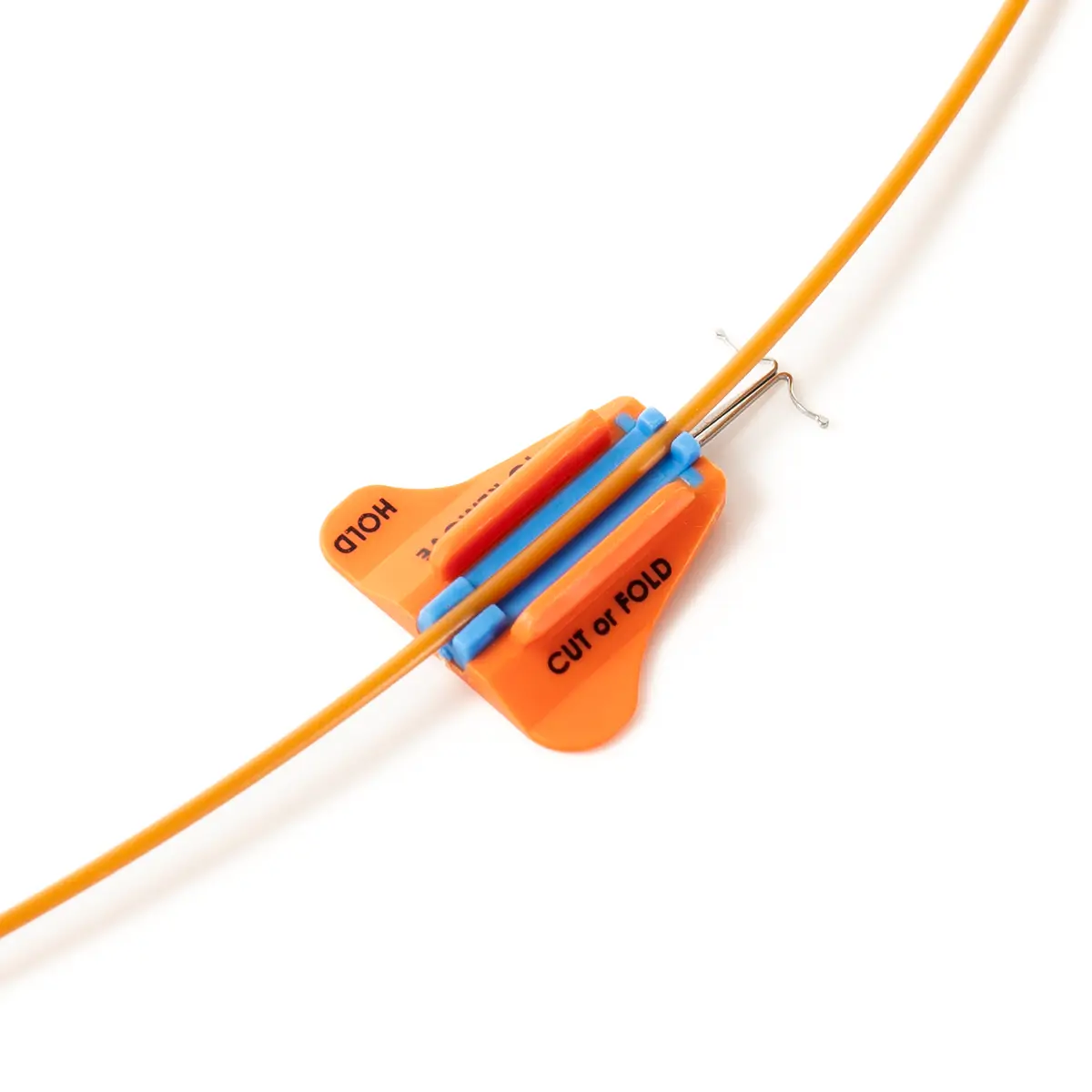
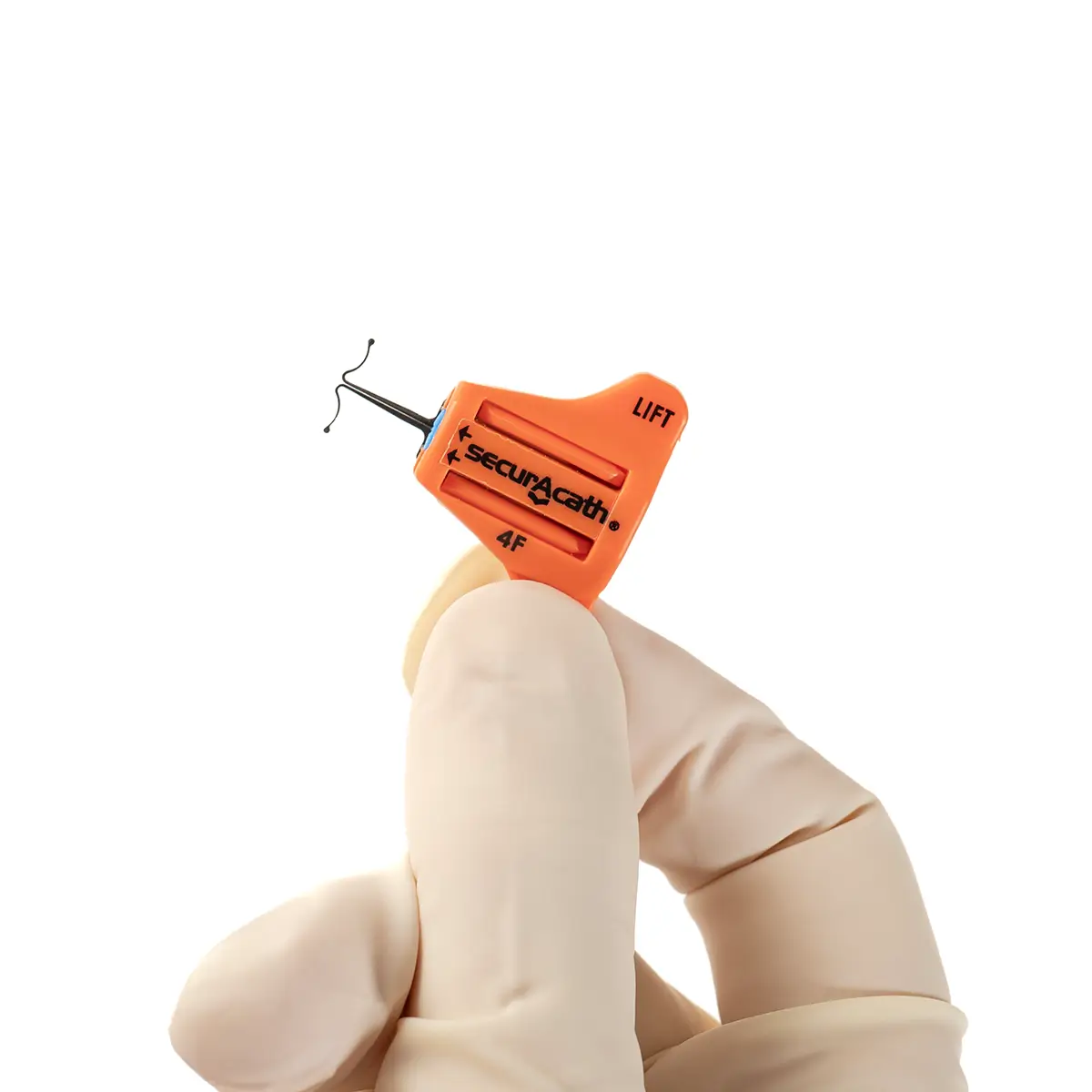
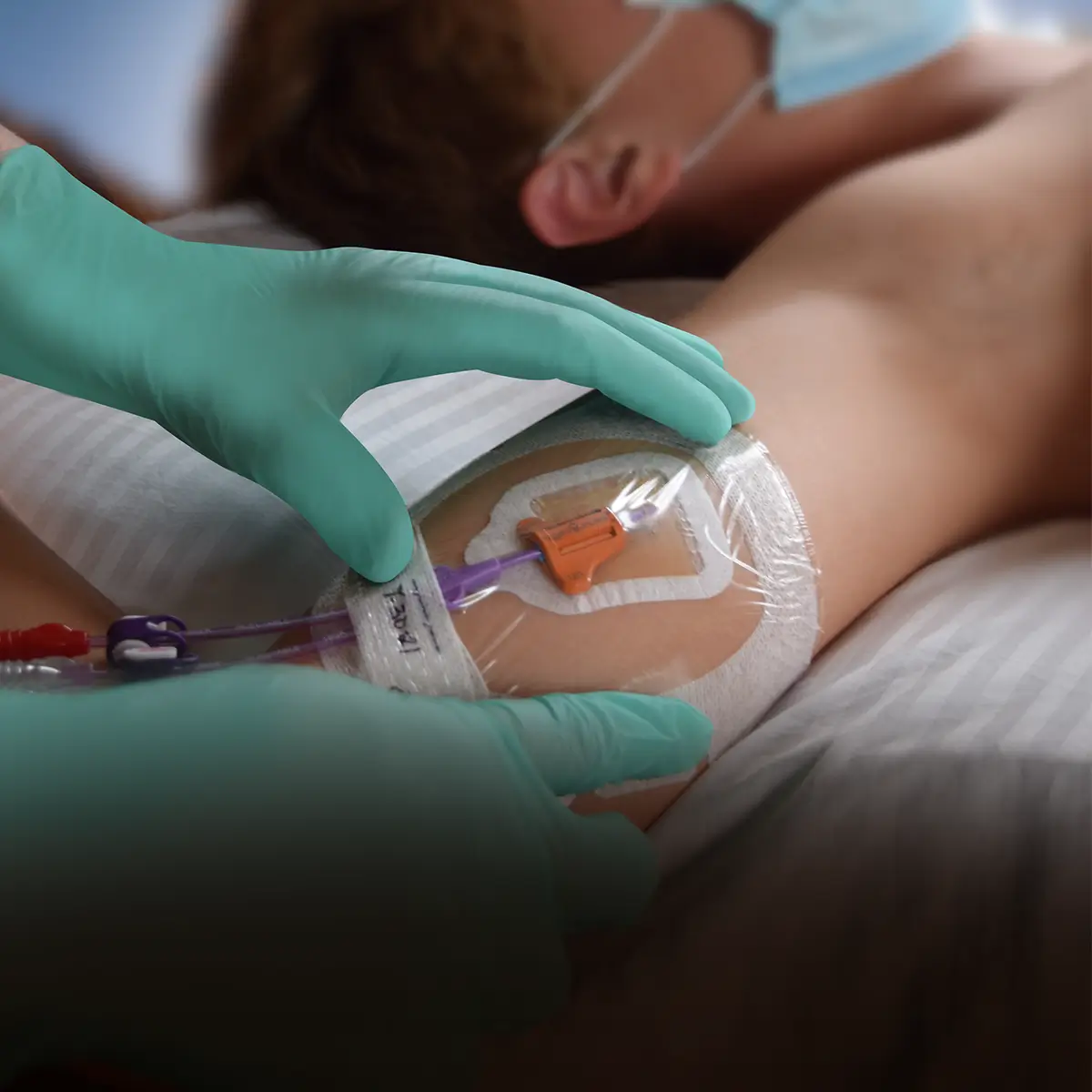
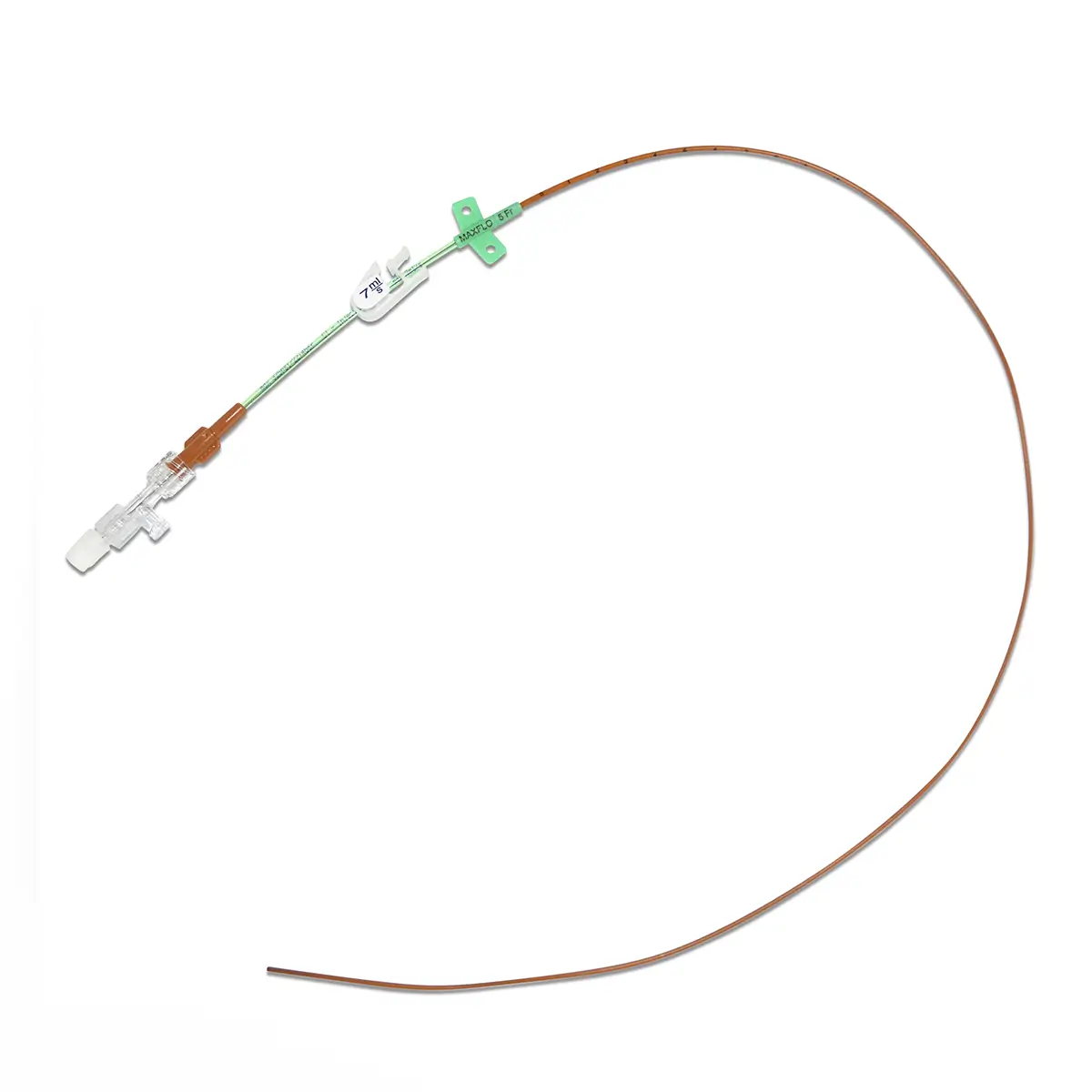
The SecurAcath® Subcutaneous Anchor Securement System (SASS) is a revolutionary new method for catheter securement that does not require sutures or adhesives. The unique design of the SecurAcath® secures right at the insertion site using small, flexible securement feet placed in the subcutaneous tissue just beneath the skin. 6
Used for securing PICC lines, CVCs and other lines for any patients, from neonates to geriatrics.
Vygon (UK) Ltd – Leading Supplier of Medical & Surgical Devices
At Vygon we have over 60 years of experience in vascular access. Our SecurAcath range means safe, clean and accurate for all patients. Explore more PICC catheters and venous access solutions or contact us for more information.
Get in touch with your local Vygon representative today to discuss SecurAcath within your department.
References:
[1] McParlan et al, “Intravascular catheter migration: A cross-sectional and health-economic comparison of adhesive and subcutaneous engineered stabilisation devices for intravascular device securement.” Journal of Vascular Access (2019):online
[2] Pittiruti M, et al. “Clinical experience of a subcutaneously anchored sutureless system for securing central venous catheters.” British Journal of Nursing (2019) Jan 24;28(2):S4-14.
[3] Zerla et al. “Evaluating Safety, Efficacy, and Cost-Effectiveness of PICC Securement by Subcutaneously Anchored Stabilization Device.” Journal of Vascular Access 18.3 (2017):238-242.
[4] Dolcino et al. “Potential Role of a Subcutaneously Anchored Securement Device in Preventing Dislodgement of Tunneled-Cuffed Central Venous Devices in Pediatric Patients.” Journal of Vascular Access 18.6 (2017):540-545.
[5] Hughes, Meinir Elen. “Reducing PICC migrations and improving patient outcomes.” British Journal of Nursing 23:Sup1, (2014): S12-S18.
[6] Rowe, et al, “Catheter Securement Impact on PICC-related CLABSI: A University Hospital Perspective” American Journal of Infection Control, Open Access, June 17, 2020
The flexible securement feet are made of Nitinol which is a shape-memory alloy of nickel and titanium. Nitinol is used in several medical devices including self-expanding stents and IVC filters. The plastic is polypropylene and elastomer. The SecurAcath® is not made with natural rubber latex.
MRI Conditional.
Product Codes (Size / Code)
- 3F VIA400130
- 4F VIA400140
- 5F VIA400110
- 6F VIA400150
- 7F VIA400120
- 8F VIA400160
- 9F VIA400170
- 10F VIA400180
- 12F VIA400200