Field Safety Notice (FSN): Peelable Cannula

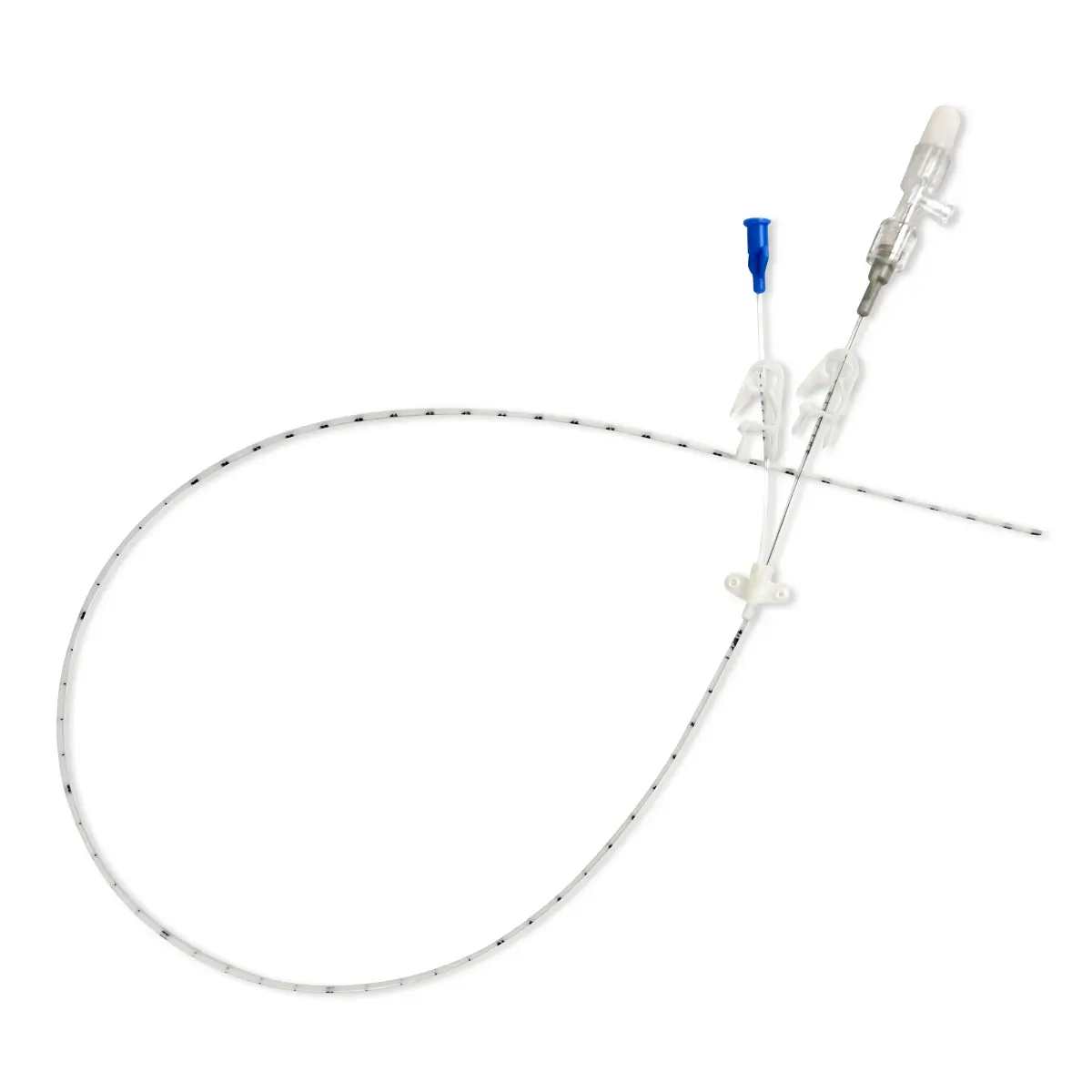
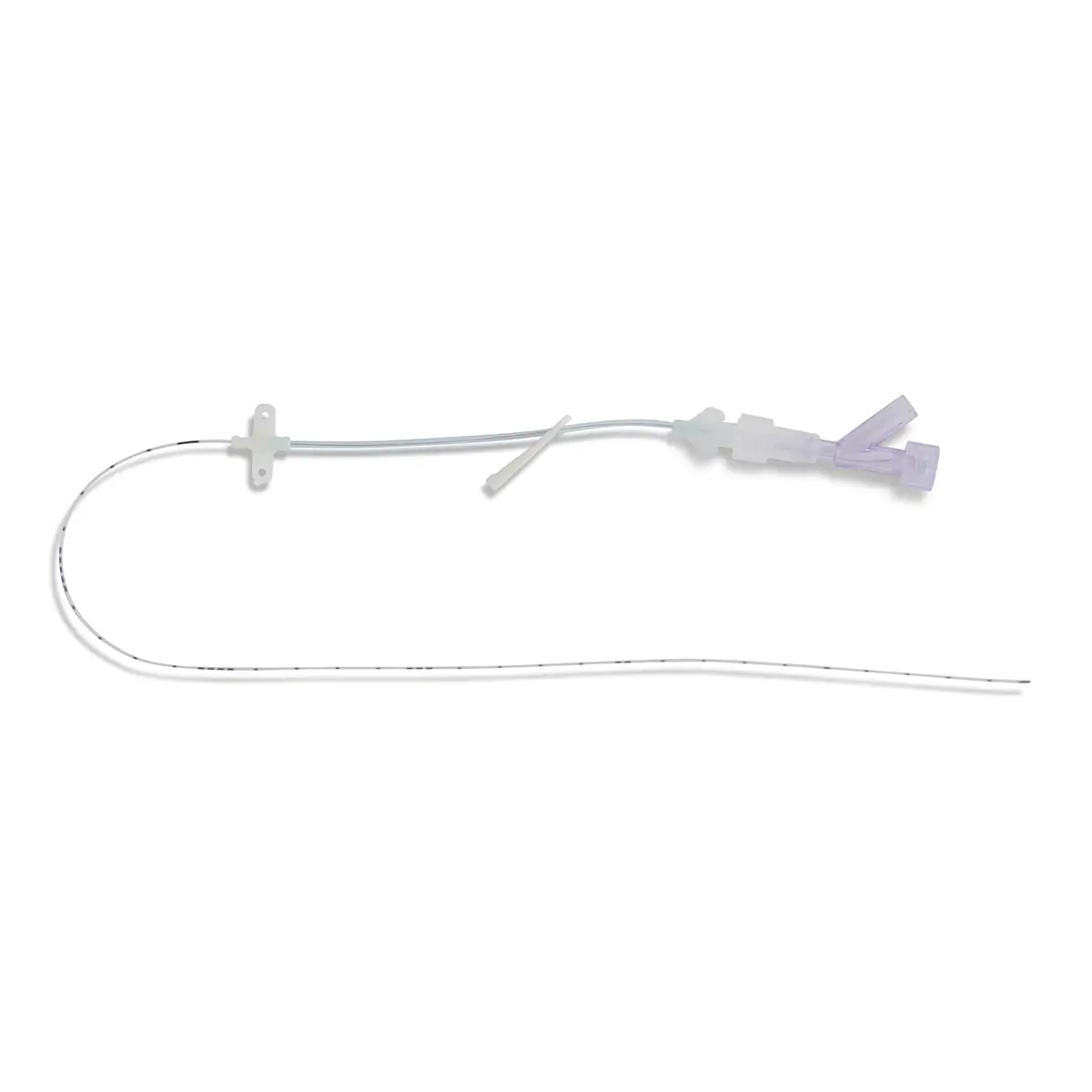
Product: Peelable Cannula
Description of the Problem:
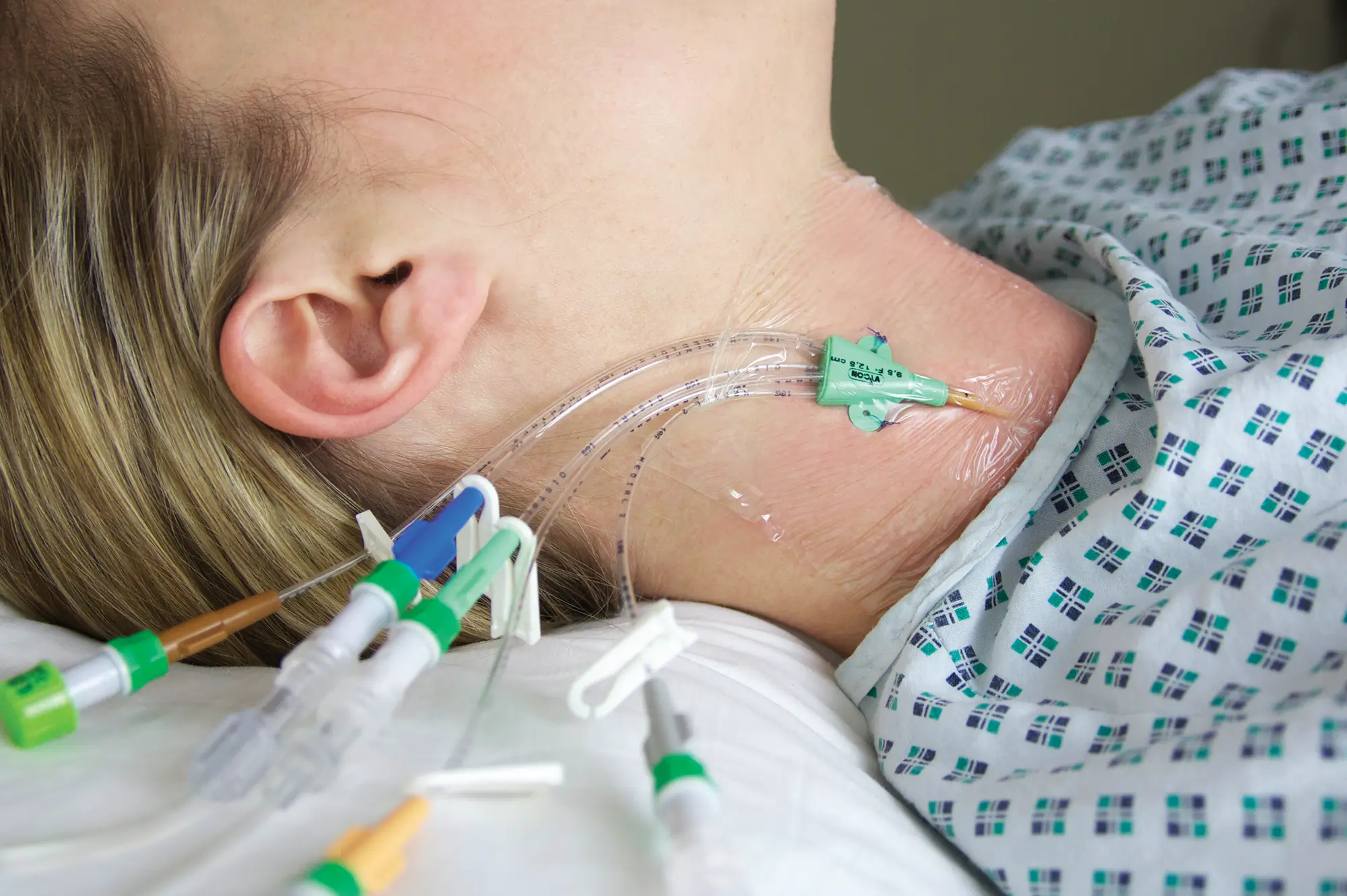
The legal manufacturer, Vygon GmbH, has identified a possible malfunction of the peelable introducer cannula included with the product codes and batch numbers listed below. After catheter placement and withdrawal of the peelable cannula, in a few cases the cannula may not peel completely, causing one half of the cannula to break off. As a result, a section of the cannula will remain on the catheter.
Affected Devices:
- 00114702 – MICROSITE 2FR MST KIT – Batch No: 060122GG
- 00114702 – MICROSITE 2FR MST KIT – Batch No: 091221GG
- 001252030 – NUTRILINE STYLETTED 24G 30CM – Batch No: 240122GP
- 001261208 – PREMICATH+STYLET+MICROFLSH INTRO B10 – Batch No: 050122GS
Action:
If this occurs, the legal manufacturer recommends that the catheter remains in situ and the non-removable section of the cannula is gently pulled along with catheter towards the hub as far as possible and secured. To improve securement, the yellow cannula wing and the peeled section of cannula may be cut off with a pair of scissions, leaving the distal part of the cannula on the catheter. Please ensure that there is sufficient distance between the catheter and scissors to prevent accidental damage to the catheter
Field Safety Notice (FSN):
Please find attached a copy of the FSN and email back form through this link.
The FSN has been sent to all customers of the affected codes.
For more information please use the Contact Form
Field Safety Notice: Peelable Cannula
Contact Us
For more information on this field safety notice, or any other queries please get in touch: