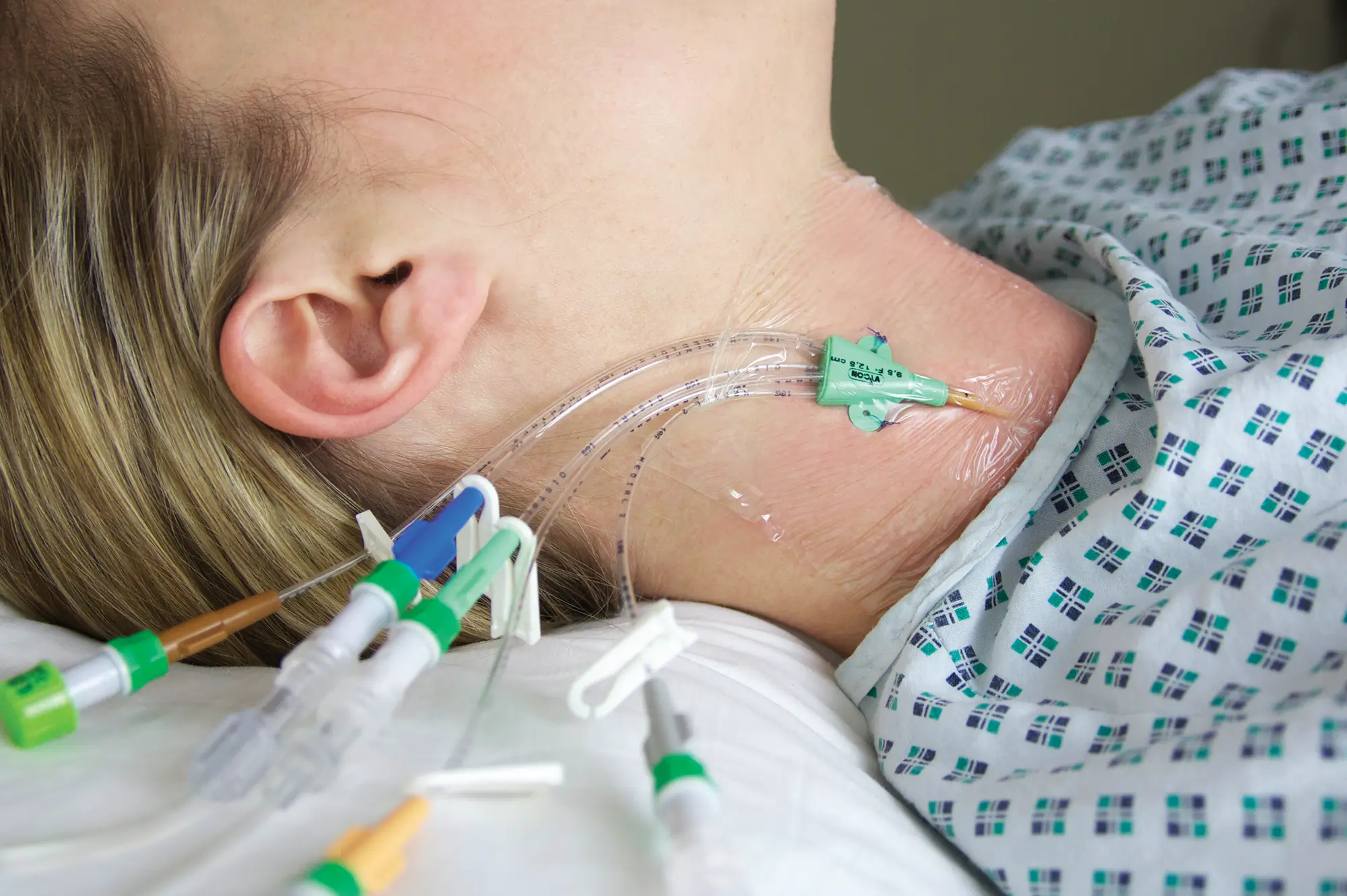
Lifecath midlines improve treatment for patients

Lifecath midlines improve treatment for patients at Gloucestershire Hospitals NHS Foundation Trust
March 2021
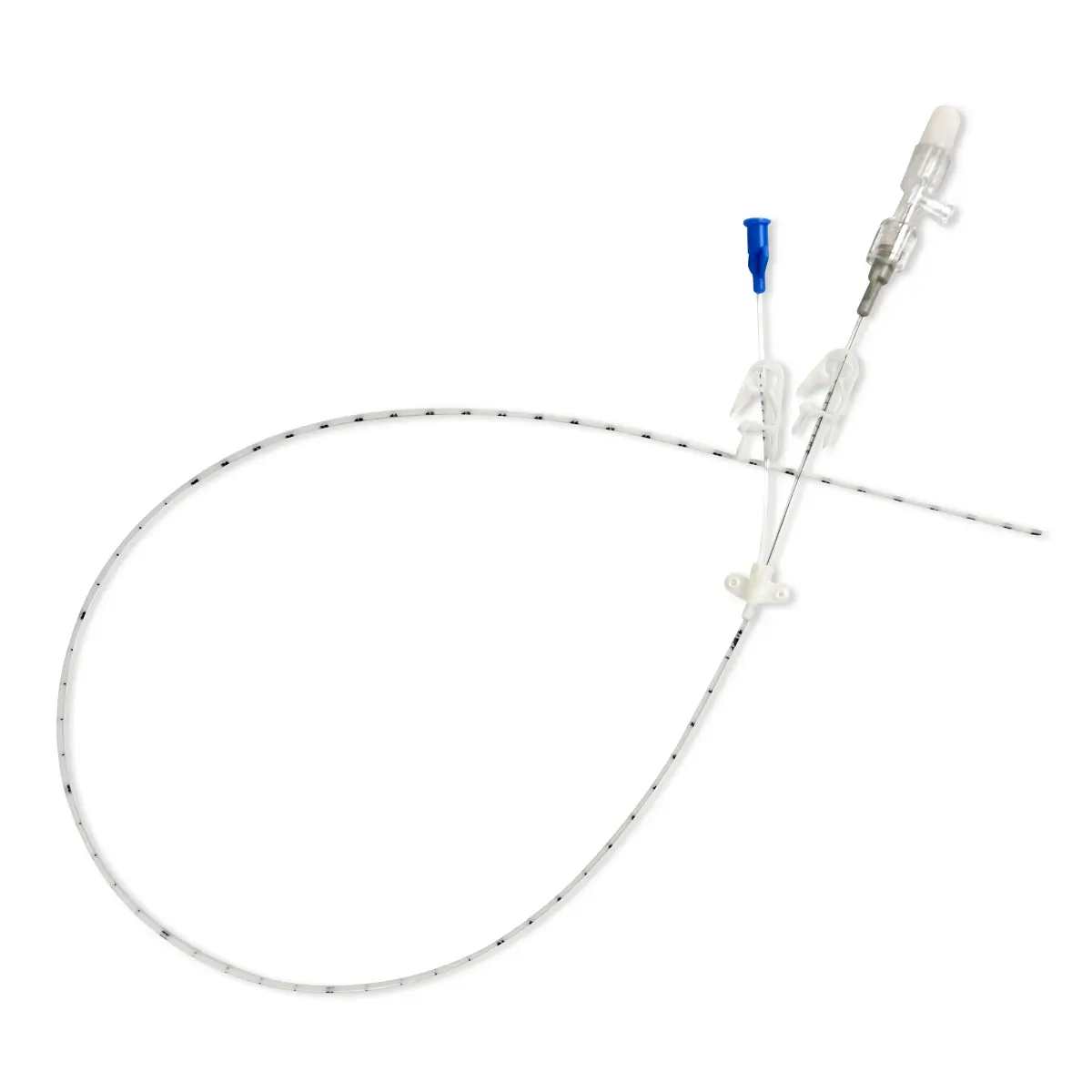
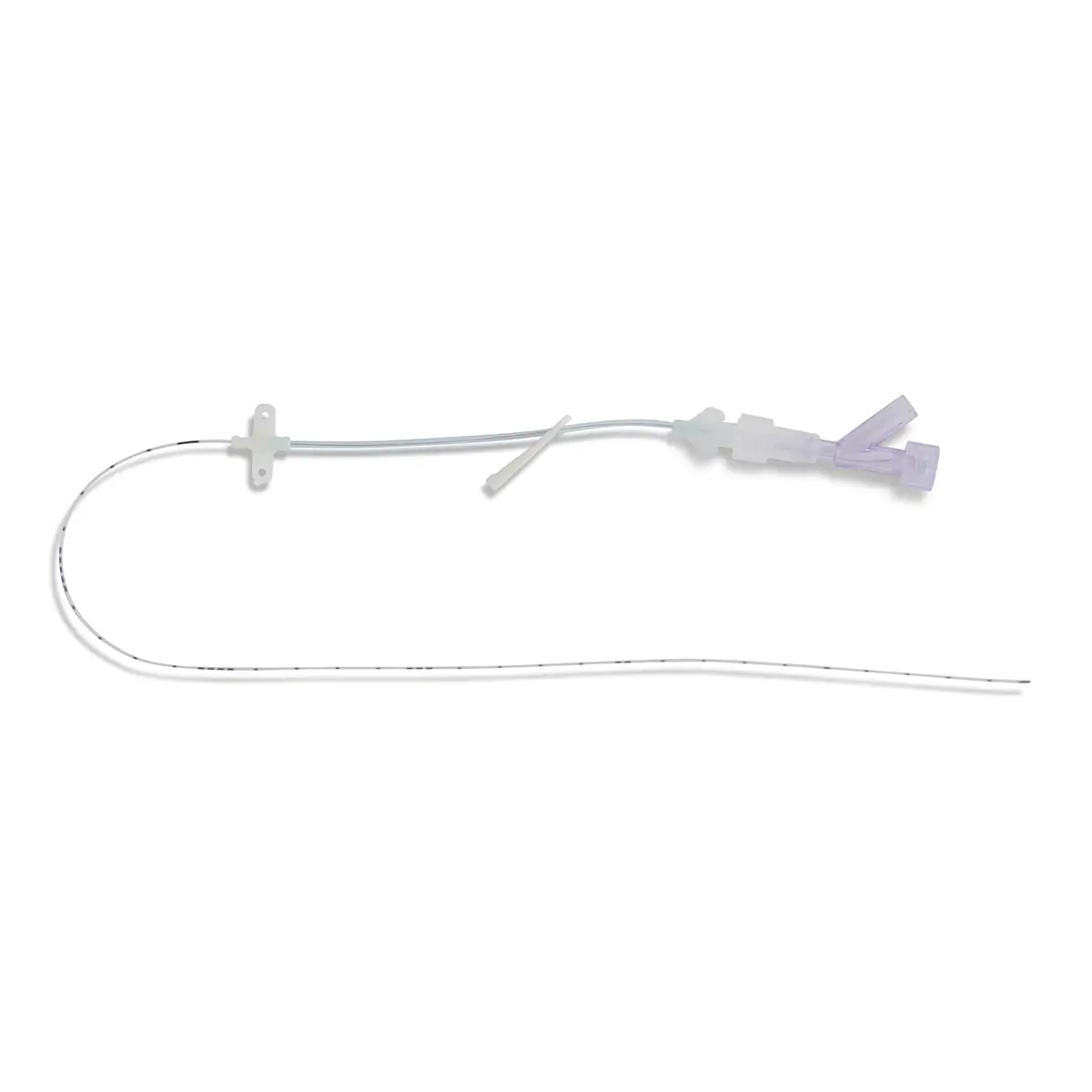
Introducing Vygon’s Lifecath midlines for difficult venous access patients has transformed the way the clinical teams at Gloucestershire Hospitals NHS Foundation Trust are able to deliver treatment.
A key benefit of using Lifecath midline is that they can be left in situ for extended periods of time and dwell time can be for the full duration of the treatment, even when it exceeds 29 days. For the clinical team in Gloucestershire, this provided an extended dwell time of up to six weeks to deliver antibiotics and other therapies. The result is a better patient experience as there is no need for repeated, painful insertions due to failure, or reduced dwell time from other devices.
Another feature of Lifecath midline valued by the Gloucestershire team is the ability to allow blood sampling; a vital function that is difficult in long peripheral catheters and impossible in short peripheral catheters. Attempts at phlebotomy with an alternative midline brand were previously unsatisfactory.
The switch to Lifecath midline was led by Julian Phelps, Advanced Nurse Practitioner at Gloucestershire NHS Trust.
The hospital had been using a CT rated 5Fr dual lumen midline from another supplier which they used for short-term antibiotic treatment on difficult venous access patients. Julian and his team had also replaced the use of some PICCs with midlines for endocarditis and bronchiectasis patients.
Unfortunately, the midlines were not lasting any longer than two weeks and were extremely unreliable for phlebotomy. Plus, although the lines were CT rated, the infusion pumps in radiology could not be used with anything other than a cannula.
Julian decided to trial Lifecath midline after attending a Vygon webinar entitled ‘Vascular Access in a post Covid-19 world: what do we learn and new paradigms’. One of the presentations was delivered by Dr. Mauro Pittiruti, Vascular Surgeon, Founder and President of GAVeCELT, and Chairman of the Scientific Committee of WoCoVA. In this presentation, Dr. Pittiruti talked about how midlines could be left in place for four weeks or longer.
Julian explains:
“The webinar raised some very interesting points and we decided to evaluate Lifecath midline for a month with 25 patients. We kept a database for recording the duration of the lines which were being used for long term antibiotic therapy both in hospital and in the community.
We found the main benefit of the Vygon Lifecath midline was the extended dwell time of six weeks for antibiotics and the reliability for blood sampling.”
Lifecath midline has also enabled a change of practice within the vascular access team to place lines. Most insertions are now by the bedside using the brachial vein with ultrasound scanning to confirm that the tip of the catheter is in the subclavian vein. Julian and his team have placed more than 100 lines to date, many of these are for antibiotic therapy and in place of PICCs.
A further benefit of the bedside insertions of Lifecath midline is the ability to give longer-term vascular access to Covid positive patients in isolation. Inserting a PICC line for these patients was not previously possible because they could not be moved around the hospital to the clinical room for placement.
The change to Lifecath midline has also resulted in cost savings. Compared to a PICC, there is a reduction of £45 per unit and although the Vygon midlines are more expensive than the previous brand used, Lifecath midline is suitable for a larger number of patients (who would have previously had PICCs) so there is a saving there too.
The Trust is now using Lifecath midline exclusively and is increasing the number of lines they place, particularly for outpatient respiratory conditions.
“Our change of practice has made a big difference in the Trust, allowing us to become more effective as a team and often allowing for fewer delays in treatment”.
Learn more
If you would like to speak to a Vygon representative regarding how our Vascular Access products can support your Trust, please complete the form below.